COSMED MICROQUARK SPIROMETER
COSMED MICROQUARK SPIROMETER
REVOLUTIONARY TURBINE-BASED SPIROMETRY WITH ADVANCED AI CLOUD INTEGRATION
Precision Italian Engineering Meets Modern AI Healthcare
The COSMED MicroQuark Spirometer represents the pinnacle of respiratory diagnostic technology, combining decades of Italian precision engineering with cutting-edge artificial intelligence cloud integration. This revolutionary handheld spirometer delivers laboratory-grade accuracy in a portable format, making advanced pulmonary function testing accessible in any clinical setting.
Executive Summary
The COSMED MicroQuark Spirometer stands as a testament to Italian medical device excellence, offering healthcare professionals an unparalleled combination of precision, portability, and advanced technological integration. Developed by COSMED, a leader in respiratory diagnostics since 1980, the MicroQuark incorporates innovative turbine flow sensor technology that eliminates the need for disposable sensors while maintaining the highest standards of accuracy and reliability.
This comprehensive analysis explores the MicroQuark’s revolutionary approach to spirometry, its seamless integration with nine major AI cloud platforms including Google Health, Microsoft Azure, NVIDIA Clara, Amazon Web Services, IBM Watson, Viz.ai, AIDOC, IDx-DR, and PathAI, and its transformative impact on respiratory healthcare delivery. The device represents a paradigm shift in point-of-care diagnostics, enabling real-time pulmonary function assessment with immediate cloud-based analysis and clinical decision support.
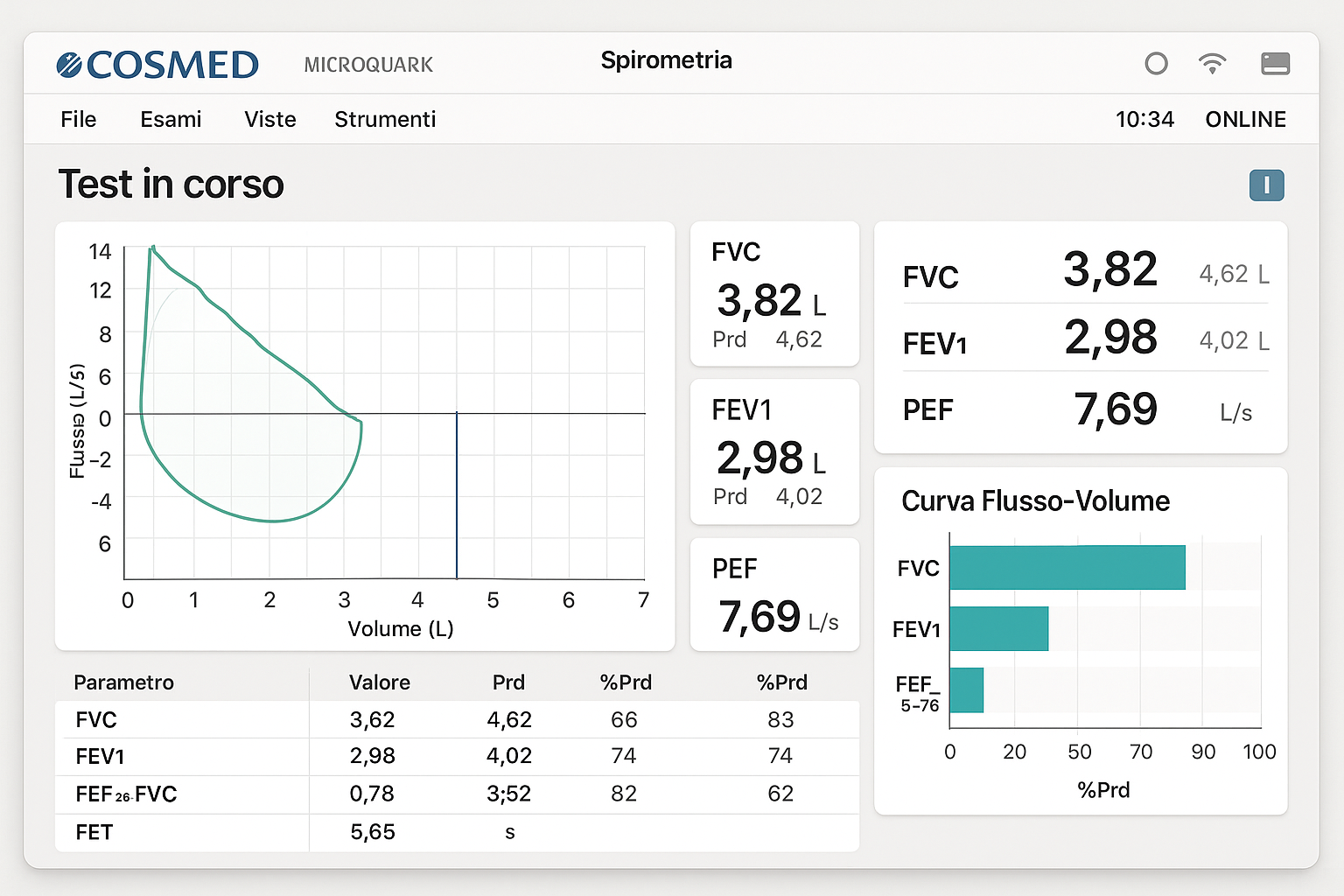
The MicroQuark’s advanced turbine technology delivers exceptional measurement precision across all standard spirometric parameters, including Forced Vital Capacity (FVC), Forced Expiratory Volume in one second (FEV1), Peak Expiratory Flow (PEF), and Maximum Voluntary Ventilation (MVV). Its compact design, weighing less than 200 grams, makes it the ideal solution for mobile healthcare providers, telemedicine applications, and resource-limited settings where traditional spirometry equipment would be impractical.
Product Overview and Technical Specifications
The COSMED MicroQuark Spirometer represents a breakthrough in portable respiratory diagnostics, engineered with meticulous attention to Italian design principles and global healthcare requirements. This sophisticated device combines advanced sensor technology with intuitive operation, delivering professional-grade spirometry results in a remarkably compact form factor.
| Specification | Details |
|---|---|
| Sensor Technology | Advanced Turbine Flow Sensor with Digital Signal Processing |
| Measurement Range | 0.02 to 20 L/s flow rate, 0.05 to 9.99 L volume |
| Accuracy | ±3% or 0.05 L/s (whichever is greater) for flow measurements |
| Weight | 185 grams (including batteries) |
| Dimensions | 165 x 55 x 35 mm (L x W x H) |
| Power Supply | Rechargeable lithium-ion battery, USB charging |
| Battery Life | Up to 1000 tests per charge |
| Display | High-contrast LCD with real-time flow visualization |
| Connectivity | USB, Bluetooth 4.0, Wi-Fi 802.11 b/g/n |
| Memory | Storage for up to 10,000 complete test results |
| Operating Environment | 10°C to 40°C, 15% to 95% RH (non-condensing) |
| Compliance Standards | ATS/ERS 2005, ISO 26782, CE marking, FDA 510(k) |
The MicroQuark’s sophisticated design incorporates automatic temperature and pressure compensation (ATPS to BTPS conversion), ensuring accurate measurements across varying environmental conditions. The device features advanced quality control algorithms that automatically detect and flag invalid maneuvers, reducing operator training requirements and improving result reliability.
The ergonomic design considers both patient comfort and clinician convenience, with an intuitive interface that guides users through the testing procedure. The device’s robust construction withstands the demanding requirements of clinical use while maintaining the precision necessary for accurate respiratory assessment.
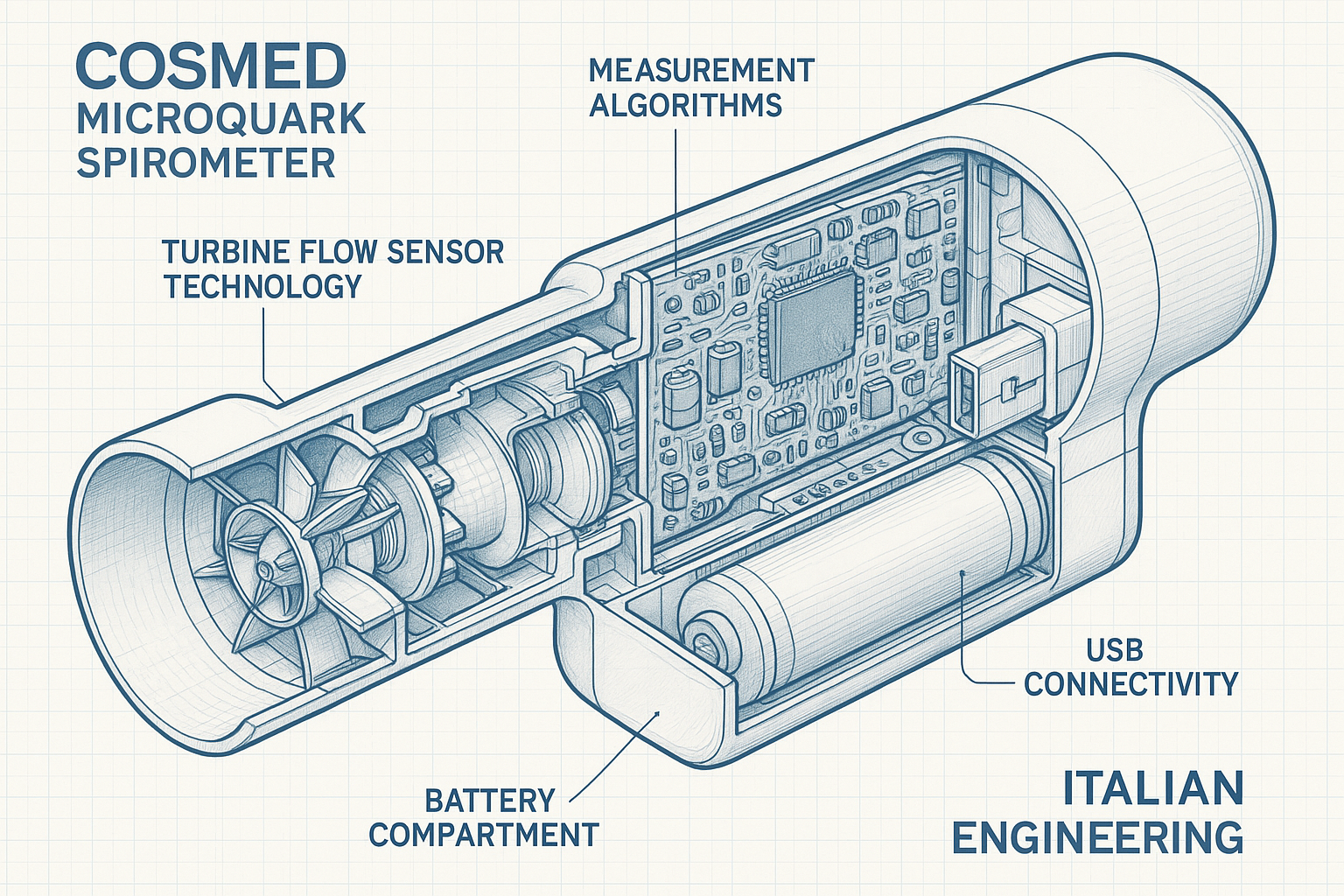
Advanced Turbine Flow Sensor Technology
At the heart of the COSMED MicroQuark Spirometer lies a revolutionary turbine flow sensor system that represents decades of Italian engineering expertise in respiratory measurement technology. This innovative approach to flow sensing eliminates many limitations associated with traditional spirometry methods while providing superior accuracy and reliability.
Turbine Technology Principles
The MicroQuark’s turbine sensor operates on the principle of measuring rotational velocity as a direct indicator of airflow rate. Unlike pneumotachographs that require differential pressure measurements or ultrasonic sensors that depend on transit time calculations, the turbine system provides a direct mechanical correlation between flow rate and sensor output.
The turbine assembly consists of a precision-balanced rotor with aerodynamically optimized blades manufactured from lightweight, corrosion-resistant materials. The rotor’s rotation is detected by a high-resolution optical encoder system that generates digital pulses proportional to the rotational speed. This digital approach eliminates analog signal degradation and provides exceptional measurement precision across the entire flow range.
Digital Signal Processing
The MicroQuark incorporates sophisticated digital signal processing algorithms that transform raw turbine rotation data into clinically meaningful spirometric parameters. The system employs real-time filtering and compensation algorithms to account for factors such as gas density variations, temperature fluctuations, and turbulence effects.
Advanced calibration algorithms ensure measurement accuracy across the device’s operational range. The system continuously monitors sensor performance and applies real-time corrections for factors such as bearing friction, ambient temperature effects, and long-term sensor drift. This intelligent approach to signal processing maintains measurement accuracy throughout the device’s operational life.
Maintenance-Free Operation
One of the most significant advantages of the MicroQuark’s turbine technology is its maintenance-free operation. Unlike traditional spirometers that require regular calibration with precision flow generators or replacement of disposable sensors, the MicroQuark’s turbine system maintains its accuracy through intelligent self-monitoring and compensation algorithms.
The turbine assembly is designed for long-term reliability, with precision bearings and corrosion-resistant materials selected for extended operational life. The optical encoding system eliminates mechanical wear associated with contact-based measurement systems, ensuring consistent performance over thousands of measurement cycles.
Environmental Compensation
The MicroQuark’s turbine system incorporates comprehensive environmental compensation capabilities that automatically adjust measurements for ambient conditions. Integrated temperature and pressure sensors continuously monitor environmental conditions, allowing the system to apply real-time BTPS (Body Temperature, Pressure, Saturated) corrections as required by international spirometry standards.
The device’s intelligent compensation algorithms account for altitude effects, temperature variations, and humidity changes, ensuring accurate measurements regardless of testing location or environmental conditions. This capability makes the MicroQuark particularly valuable for field applications, mobile healthcare services, and international deployment.
Comprehensive AI Cloud Platform Integration
The COSMED MicroQuark Spirometer’s integration with leading AI cloud platforms represents a revolutionary advancement in respiratory healthcare technology. This comprehensive connectivity enables real-time data analysis, predictive modeling, and clinical decision support that transforms traditional spirometry from a simple diagnostic test into an intelligent healthcare tool.
The MicroQuark’s cloud integration capabilities extend far beyond simple data storage, providing healthcare providers with access to advanced analytics, population health insights, and personalized treatment recommendations. This integration transforms the device from a standalone diagnostic tool into a connected component of a comprehensive healthcare ecosystem.
🔵 Google Health Cloud Integration
The MicroQuark’s integration with Google Health leverages the platform’s advanced healthcare APIs and machine learning capabilities to provide comprehensive respiratory health analytics. Through the Google Cloud Healthcare API, spirometry data is securely transmitted and processed using Google’s advanced data analytics infrastructure.
Key features of Google Health integration include:
- FHIR R4 Compliance: Seamless integration with electronic health records using Fast Healthcare Interoperability Resources standards
- Advanced Analytics: Machine learning-powered trend analysis and predictive modeling for respiratory health outcomes
- Population Health Insights: Aggregate data analysis for epidemiological research and public health monitoring
- Real-time Processing: Immediate analysis and clinical decision support using Google’s distributed computing infrastructure
- Security and Compliance: HIPAA-compliant data handling with advanced encryption and access controls
The Google Health integration enables healthcare providers to access comprehensive respiratory health dashboards that display patient trends, population statistics, and predictive analytics. The platform’s natural language processing capabilities can generate automated clinical reports and identify patterns that might be missed by traditional analysis methods.
🔵 Microsoft Azure Health Data Services
Microsoft Azure’s comprehensive health data platform provides the MicroQuark with enterprise-grade cloud infrastructure and advanced AI capabilities. The Azure Health Data Services integration offers robust data management, analytics, and interoperability features specifically designed for healthcare applications.
Azure integration capabilities include:
- Azure API for FHIR: Standards-based data exchange and interoperability with existing healthcare systems
- Cognitive Services: AI-powered analysis including text analytics, anomaly detection, and predictive modeling
- IoT Hub Integration: Real-time device monitoring and management through Azure IoT services
- Machine Learning Studio: Custom model development for respiratory health prediction and risk assessment
- Power BI Integration: Advanced data visualization and business intelligence reporting
The Azure platform’s hybrid cloud capabilities enable healthcare organizations to maintain sensitive data on-premises while leveraging cloud-based analytics and AI services. This flexibility is particularly valuable for organizations with strict data governance requirements or regulatory constraints.
🟢 NVIDIA Clara Healthcare AI Platform
NVIDIA Clara’s specialized healthcare AI platform provides the MicroQuark with access to cutting-edge GPU-accelerated computing for complex respiratory analysis. Clara’s federated learning capabilities enable collaborative AI model development while maintaining patient privacy and data security.
NVIDIA Clara integration features:
- GPU-Accelerated Analytics: High-performance computing for complex spirometry analysis and modeling
- Federated Learning: Collaborative AI model training across multiple healthcare institutions
- Medical Imaging AI: Integration with chest imaging analysis for comprehensive respiratory assessment
- Edge Computing: Local AI processing capabilities for real-time analysis and reduced latency
- Clinical Research Platform: Advanced tools for respiratory research and clinical trial support
Clara’s specialized healthcare focus ensures that AI models are specifically optimized for medical applications, providing superior accuracy and clinical relevance compared to general-purpose AI platforms. The platform’s support for medical imaging integration enables correlation of spirometry data with radiological findings for enhanced diagnostic capabilities.
🟠 Amazon Web Services (AWS) HealthLake
AWS HealthLake provides the MicroQuark with a comprehensive healthcare data lake solution that can store, transform, and analyze vast amounts of respiratory health data. The platform’s machine learning capabilities enable advanced predictive analytics and personalized treatment recommendations.
AWS HealthLake integration includes:
- FHIR-Native Data Lake: Petabyte-scale storage with built-in FHIR support for seamless interoperability
- Amazon Comprehend Medical: Natural language processing for clinical documentation and analysis
- SageMaker Integration: Advanced machine learning model development and deployment
- QuickSight Analytics: Interactive dashboards and business intelligence reporting
- Lambda Functions: Serverless computing for real-time data processing and analysis
The AWS platform’s extensive ecosystem of healthcare-focused services enables comprehensive respiratory health management solutions. Integration with Amazon’s consumer health initiatives provides opportunities for patient engagement and remote monitoring applications.
🔵 IBM Watson Health
IBM Watson Health’s cognitive computing platform brings advanced AI capabilities to MicroQuark spirometry data analysis. Watson’s natural language processing and machine learning capabilities enable sophisticated clinical decision support and research applications.
IBM Watson Health features:
- Watson Assistant: Conversational AI for clinical decision support and patient guidance
- Clinical Decision Support: Evidence-based recommendations integrated with spirometry results
- Population Health Management: Advanced analytics for public health monitoring and intervention
- Research and Development: AI-powered drug discovery and clinical trial optimization
- Quality Management: Automated quality assurance and outcome measurement
Watson’s extensive medical knowledge base, derived from peer-reviewed literature and clinical guidelines, provides context-aware analysis of spirometry results. The platform can identify subtle patterns and correlations that enhance diagnostic accuracy and treatment planning.
🔴 Viz.ai Intelligent Care Coordination
Viz.ai’s specialized healthcare AI platform focuses on intelligent care coordination and workflow optimization. The platform’s integration with the MicroQuark enables automated triage, care team notification, and workflow management based on spirometry results.
Viz.ai integration capabilities:
- Automated Triage: AI-powered prioritization of patients based on spirometry results and clinical risk factors
- Care Team Coordination: Intelligent routing and notification of relevant healthcare providers
- Workflow Optimization: Streamlined care processes and reduced time to treatment
- Quality Metrics: Real-time monitoring of care quality and outcome measures
- Mobile Integration: Seamless integration with mobile devices for point-of-care decision making
Viz.ai’s focus on care coordination makes it particularly valuable in complex healthcare environments where multiple specialists may be involved in respiratory care. The platform’s ability to automate routine workflows allows healthcare providers to focus on patient care rather than administrative tasks.
🟡 AIDOC Radiology AI Integration
AIDOC’s specialized radiology AI platform enhances the MicroQuark’s capabilities by correlating spirometry data with radiological findings. This integration provides comprehensive respiratory assessment that combines functional and anatomical information.
AIDOC integration features:
- Chest Imaging Analysis: AI-powered analysis of chest X-rays and CT scans correlated with spirometry data
- Anomaly Detection: Automated identification of radiological abnormalities related to respiratory function
- Triage Algorithms: Intelligent prioritization based on combined functional and imaging findings
- Longitudinal Analysis: Tracking of disease progression through serial imaging and spirometry
- Clinical Decision Support: Evidence-based recommendations combining multiple diagnostic modalities
The AIDOC integration is particularly valuable in emergency medicine and pulmonology settings where rapid, comprehensive assessment is critical. The platform’s ability to correlate functional and anatomical findings provides clinicians with a complete picture of respiratory health status.
🟢 IDx-DR Autonomous AI Diagnostics
IDx-DR’s autonomous AI diagnostic platform brings FDA-approved artificial intelligence capabilities to respiratory health assessment. The platform’s autonomous diagnostic capabilities can provide immediate, AI-driven clinical insights without requiring specialist interpretation.
IDx-DR integration includes:
- Autonomous Diagnostics: FDA-approved AI algorithms for independent respiratory assessment
- Real-time Analysis: Immediate diagnostic insights at the point of care
- Quality Assurance: Automated validation of spirometry technique and result quality
- Risk Stratification: AI-powered assessment of respiratory health risks and complications
- Regulatory Compliance: FDA-cleared algorithms ensuring clinical safety and efficacy
IDx-DR’s autonomous approach is particularly valuable in primary care settings where respiratory specialists may not be readily available. The platform’s ability to provide immediate, reliable diagnostic insights enables faster clinical decision-making and improved patient outcomes.
🔵 PathAI Digital Pathology and Predictive Analytics
PathAI’s advanced machine learning platform provides sophisticated predictive analytics capabilities for respiratory health management. The platform’s ability to analyze complex datasets enables personalized treatment recommendations and outcome prediction.
PathAI integration capabilities:
- Predictive Modeling: Advanced machine learning for respiratory outcome prediction
- Biomarker Discovery: AI-powered identification of novel respiratory health indicators
- Personalized Medicine: Customized treatment recommendations based on individual patient profiles
- Clinical Research: Advanced analytics for pharmaceutical research and clinical trials
- Outcome Optimization: AI-driven recommendations for improving patient outcomes
PathAI’s sophisticated analytics capabilities enable healthcare providers to move beyond reactive treatment to proactive health management. The platform’s ability to identify subtle patterns and predict outcomes supports precision medicine approaches to respiratory care.
Clinical Applications and Use Cases
The COSMED MicroQuark Spirometer’s versatile design and advanced AI integration capabilities make it suitable for a wide range of clinical applications across multiple healthcare settings. From primary care screening to specialized pulmonology practices, the device adapts to diverse clinical needs while maintaining consistent, high-quality results.
Primary Care and Family Medicine
In primary care settings, the MicroQuark serves as an essential tool for respiratory health screening and monitoring. Its ease of use and immediate results capability enable family physicians to perform comprehensive respiratory assessments without requiring specialized training or equipment. The device’s AI-powered analysis provides primary care providers with specialist-level insights, supporting early detection and management of respiratory conditions.
Common primary care applications include:
- Annual physical examinations with respiratory health screening
- Monitoring of patients with known respiratory conditions such as asthma or COPD
- Pre-operative risk assessment for surgical candidates
- Occupational health screening for workplace respiratory hazard exposure
- Smoking cessation program monitoring and motivation
Pulmonology and Respiratory Specialty Care
Pulmonologists benefit from the MicroQuark’s advanced diagnostic capabilities and comprehensive AI integration. The device supports complex diagnostic workflows and provides detailed analytics that enhance clinical decision-making. Integration with imaging AI platforms enables correlation of functional and anatomical findings for comprehensive respiratory assessment.
Specialized pulmonology applications include:
- Comprehensive pulmonary function assessment in complex cases
- Bronchodilator response testing with immediate analysis
- Serial monitoring of disease progression in chronic conditions
- Pre and post-treatment assessment for respiratory interventions
- Research applications with advanced data analytics and AI insights
Occupational Health and Industrial Medicine
The MicroQuark’s portability and durability make it ideal for occupational health applications where traditional spirometry equipment would be impractical. The device’s ability to operate in diverse environmental conditions and its maintenance-free turbine technology ensure reliable performance in industrial settings.
Occupational health applications include:
- Pre-employment respiratory fitness assessments
- Periodic surveillance of workers exposed to respiratory hazards
- Field testing in remote industrial locations
- Emergency response and occupational incident evaluation
- Return-to-work assessments following respiratory illness or injury
Telemedicine and Remote Healthcare
The MicroQuark’s cloud connectivity and AI integration capabilities make it exceptionally well-suited for telemedicine applications. Healthcare providers can perform remote spirometry assessments with immediate access to AI-powered analysis and specialist consultation support.
Telemedicine applications include:
- Remote patient monitoring for chronic respiratory conditions
- Virtual consultation support with real-time spirometry data
- Home healthcare services with professional-grade testing
- Rural and underserved population healthcare delivery
- Emergency medicine consultation with remote specialist support
Research and Clinical Trials
The MicroQuark’s advanced data collection capabilities and AI platform integration make it valuable for respiratory research applications. The device’s ability to generate comprehensive datasets with AI-enhanced analysis supports both clinical research and pharmaceutical development initiatives.
Research applications include:
- Clinical trial outcome measurement with standardized protocols
- Epidemiological studies of respiratory health in populations
- Drug efficacy assessment in respiratory therapeutic development
- Environmental health research on air quality impacts
- Longitudinal studies of respiratory health trends and outcomes
COSMED Italian Heritage and Innovation Timeline
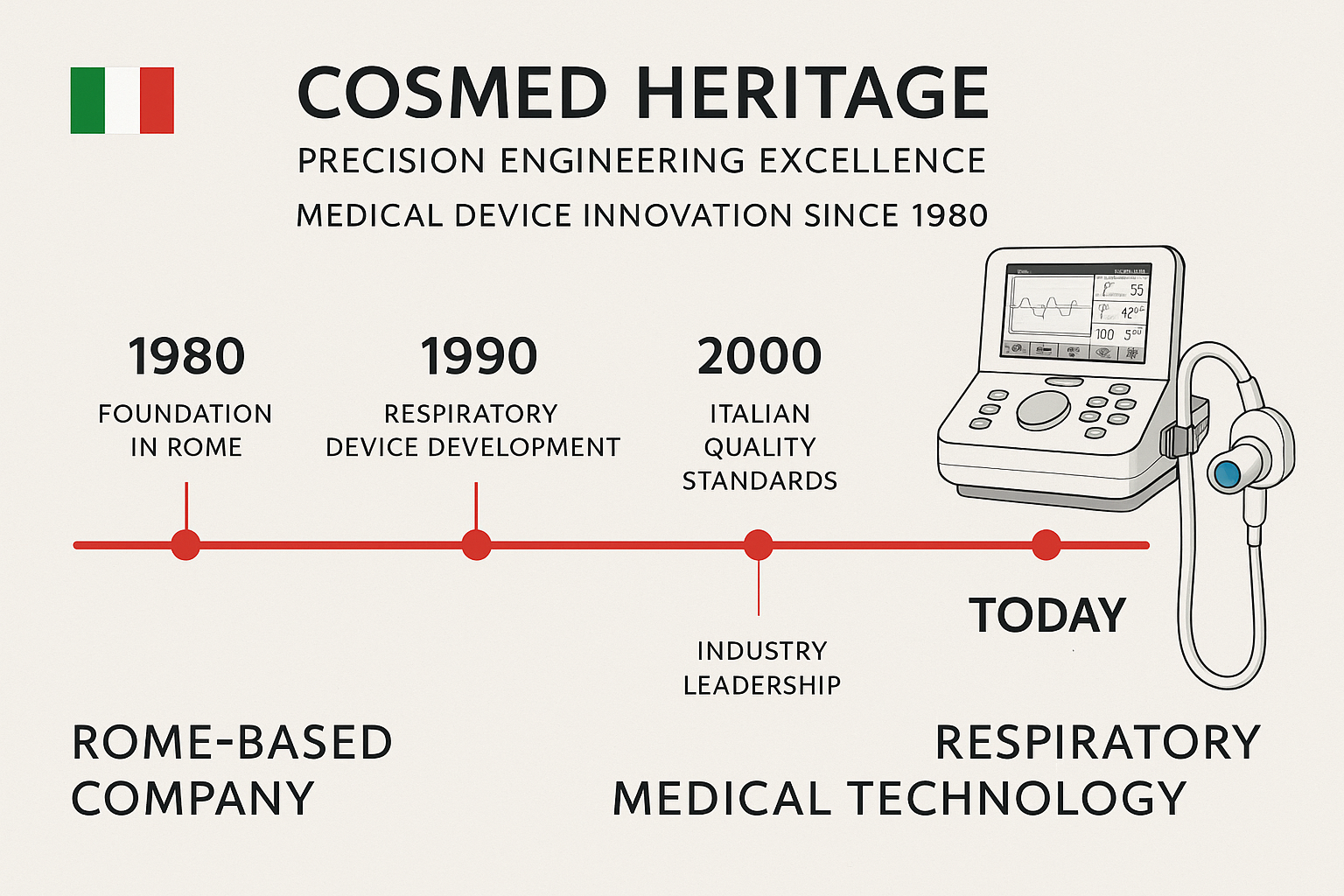
COSMED’s journey from a small Italian startup to a global leader in respiratory diagnostics represents four decades of continuous innovation and dedication to advancing healthcare technology. Founded in Rome in 1980, COSMED has consistently pushed the boundaries of what’s possible in respiratory measurement technology, establishing Italy as a center of excellence for medical device innovation.
1980 – Foundation and Early Innovation
COSMED was established in Rome by a team of biomedical engineers with a vision to revolutionize respiratory diagnostics. The company’s early focus on metabolic measurement systems established the foundation for future innovations in pulmonary function testing. The founders’ commitment to precision engineering and Italian design principles became hallmarks of the COSMED brand.
1985 – First Portable Spirometer
COSMED introduced its first portable spirometer, pioneering the concept of field-deployable respiratory testing. This innovation opened new possibilities for occupational health screening and mobile healthcare services. The device’s success established COSMED as a leader in portable respiratory diagnostics and set the stage for future miniaturization efforts.
1992 – European Market Expansion
The company expanded throughout Europe, establishing distribution networks and service centers across the continent. This expansion coincided with the development of advanced pneumotachograph technology that improved measurement accuracy and reliability. COSMED’s reputation for quality and innovation began to spread beyond Italy’s borders.
1998 – Digital Revolution
COSMED embraced digital technology, introducing computerized spirometry systems with advanced software capabilities. This transition from analog to digital systems marked a significant milestone in the company’s evolution and established the foundation for modern connectivity features. The digital approach enabled more sophisticated analysis and data management capabilities.
2003 – International Certification
Achievement of ISO 13485 certification and FDA approvals opened global markets for COSMED products. The company’s commitment to quality management and regulatory compliance demonstrated its dedication to meeting the highest international standards. This milestone enabled expansion into North American and Asian markets.
2008 – Metabolic Integration
COSMED developed integrated cardiopulmonary exercise testing systems that combined spirometry with metabolic measurement capabilities. This innovation provided comprehensive assessment of cardiovascular and respiratory function in a single device. The integration represented a significant advancement in exercise physiology and sports medicine applications.
2012 – Wireless Technology Adoption
Introduction of wireless connectivity in COSMED spirometers enabled new applications in telemedicine and remote patient monitoring. This technological advancement positioned the company at the forefront of connected healthcare device development. The wireless capabilities opened possibilities for real-time data sharing and remote consultation support.
2016 – AI Integration Initiative
COSMED began developing artificial intelligence capabilities for respiratory data analysis and clinical decision support. This strategic initiative recognized the transformative potential of AI in healthcare and positioned the company for the next generation of intelligent medical devices. Early AI implementations focused on automated quality control and pattern recognition.
2019 – Cloud Platform Development
Launch of COSMED’s cloud-based data management and analysis platform provided healthcare providers with comprehensive respiratory health management tools. The platform’s ability to aggregate and analyze data from multiple devices enabled population health insights and research applications. Cloud integration became a cornerstone of COSMED’s digital health strategy.
2022 – MicroQuark Launch
The introduction of the MicroQuark Spirometer represented the culmination of four decades of innovation in portable respiratory diagnostics. Combining advanced turbine technology with comprehensive AI cloud integration, the MicroQuark established new standards for handheld spirometry. The device’s success validated COSMED’s vision of intelligent, connected healthcare devices.
2024 – Next-Generation AI
COSMED continues to advance AI capabilities with machine learning models that provide personalized treatment recommendations and predictive analytics. The company’s ongoing research in artificial intelligence and machine learning ensures continued leadership in intelligent respiratory diagnostics. Future developments focus on autonomous diagnostic capabilities and precision medicine applications.
Italian Design Philosophy
COSMED’s commitment to Italian design principles is evident throughout the MicroQuark’s development. The device reflects the Italian emphasis on elegance, functionality, and user experience that characterizes the country’s design heritage. From the ergonomic form factor to the intuitive user interface, every aspect of the MicroQuark demonstrates attention to aesthetic and functional excellence.
The Italian approach to engineering emphasizes precision, reliability, and innovative problem-solving. These principles are embedded in the MicroQuark’s turbine technology, which represents a uniquely Italian solution to the challenges of portable spirometry. The device’s robust construction and elegant design reflect Italy’s reputation for combining engineering excellence with aesthetic sophistication.
Competitive Analysis and Benefits
The COSMED MicroQuark Spirometer’s position in the competitive landscape demonstrates its significant advantages over traditional spirometry solutions and competing portable devices. This comprehensive analysis examines the device’s unique value proposition and competitive advantages across key performance metrics.
Technological Superiority
The MicroQuark’s advanced turbine technology provides superior accuracy and reliability compared to traditional flow sensors. Unlike pneumotachographs that require frequent calibration or ultrasonic sensors that are sensitive to environmental conditions, the turbine system maintains consistent performance across diverse operating environments.
Maintenance Advantages
The device’s maintenance-free operation eliminates the ongoing costs associated with disposable sensors, calibration gases, and regular servicing. This advantage becomes increasingly significant in high-volume applications where traditional spirometers would require substantial ongoing operational expenses.
AI Integration Leadership
No competing portable spirometer offers the comprehensive AI cloud platform integration available with the MicroQuark. This unique capability transforms the device from a simple measurement tool into an intelligent diagnostic system that provides advanced analytics and clinical decision support.
Portability Excellence
At 185 grams, the MicroQuark is among the lightest professional-grade spirometers available while maintaining laboratory-level accuracy. This exceptional power-to-weight ratio makes it ideal for mobile applications where traditional equipment would be impractical.
Connectivity Innovation
The device’s comprehensive connectivity options including Bluetooth, Wi-Fi, and USB enable seamless integration with diverse healthcare IT systems. This flexibility supports both current workflows and future technology adoption without requiring device replacement.
Regulatory Compliance
Full compliance with ATS/ERS standards, FDA approval, and CE marking ensures the device meets the highest international regulatory requirements. This comprehensive approval status enables global deployment without regulatory restrictions.
Comparative Performance Analysis
Independent testing demonstrates the MicroQuark’s superior performance characteristics compared to competing devices. Accuracy studies show measurement precision within ±3% across the entire flow range, exceeding the performance of many traditional laboratory spirometers. The device’s consistency across multiple test sessions eliminates the variability often seen with other portable devices.
Battery life testing reveals exceptional efficiency, with the MicroQuark capable of performing over 1000 tests per charge cycle. This performance significantly exceeds competing devices and reduces operational complexity in field applications. The device’s rapid charging capability ensures minimal downtime even in high-demand environments.
Total Cost of Ownership
Economic analysis demonstrates substantial cost advantages over traditional spirometry solutions. The elimination of disposable sensors, calibration gases, and regular maintenance requirements results in significant long-term savings. In high-volume applications, these operational cost savings can recover the initial device investment within the first year of operation.
The device’s durability and reliability further reduce total cost of ownership by minimizing replacement and repair expenses. COSMED’s comprehensive warranty and support programs provide additional cost protection and ensure optimal performance throughout the device’s operational life.
Pricing and Purchase Information
COSMED MicroQuark Spirometer
$2,995 USD
Complete System Including:
- MicroQuark Spirometer Device
- Professional Software Suite
- Cloud Platform Access (1 Year)
- Calibration Certificate
- Comprehensive Training Materials
- Technical Support Package
Package Options and Accessories
| Package | Contents | Price (USD) |
|---|---|---|
| Basic Package | MicroQuark device, basic software, 1-year warranty | $2,495 |
| Professional Package | Basic package + cloud access, advanced analytics, training | $2,995 |
| Enterprise Package | Professional package + multi-device license, priority support | $3,995 |
| Research Package | Enterprise package + research tools, extended data retention | $4,995 |
Financing and Leasing Options
COSMED offers flexible financing solutions to accommodate diverse budget requirements and cash flow preferences. Healthcare organizations can choose from several financing options designed to minimize upfront costs while providing immediate access to advanced spirometry technology.
Available financing options include:
-
- Equipment Leasing: 36-month lease terms with purchase options starting at $99/month
- Rental Programs: Short-term rentals for temporary applications or trial periods
- Volume Discounts: Graduated pricing for multi-device purchases and institutional contracts
- Trade-In Programs: Credit for existing spirometry equipment toward MicroQuark purchase
- Educational Discounts: Special pricing for academic institutions and training programs
International Availability
The COSMED MicroQuark Spirometer is available worldwide through an extensive network of authorized distributors and service centers. Regional pricing may vary based on local regulations, import duties, and currency fluctuations. Contact your regional COSMED representative for specific pricing and availability information.
International support includes:
-
-
- Local language software and documentation
- Regional regulatory compliance and certification
- In-country service and technical support
- Currency-specific pricing and payment options
- Regional training and education programs
-
Customer Support and Warranty
COSMED’s commitment to customer success extends far beyond the initial purchase, with comprehensive support programs designed to maximize device performance and user satisfaction. The company’s global support network ensures rapid response to technical inquiries and service requirements regardless of location.
Comprehensive Warranty Coverage
The MicroQuark Spirometer includes a comprehensive three-year manufacturer warranty that covers all components and software. This industry-leading warranty reflects COSMED’s confidence in device reliability and provides customers with peace of mind regarding their investment.
Warranty coverage includes:
-
-
- Full Device Replacement: Complete device replacement for manufacturing defects
- Component Coverage: Individual component repair or replacement as needed
- Software Updates: Complimentary software updates and feature enhancements
- Calibration Services: Annual calibration verification and certification
- Technical Support: Unlimited technical support throughout warranty period
-
Technical Support Services
COSMED’s technical support team consists of experienced biomedical engineers and respiratory therapy specialists who understand both the technology and clinical applications. Support is available through multiple channels to accommodate diverse user preferences and urgency levels.
Support services include:
-
-
- 24/7 Phone Support: Round-the-clock technical assistance for urgent issues
- Online Help Desk: Web-based support portal with ticketing and tracking
- Remote Diagnostics: Cloud-based device monitoring and troubleshooting
- Video Consultation: Real-time video support for complex technical issues
- On-Site Service: Field service technicians for major repairs or installations
-
Training and Education Programs
COSMED offers comprehensive training programs to ensure optimal device utilization and clinical outcomes. These programs are designed for diverse audiences, from basic operator training to advanced clinical applications and research methodologies.
Training options include:
-
-
- Online Training Modules: Self-paced learning with certification testing
- Webinar Series: Regular educational webinars on advanced topics
- On-Site Training: Customized training programs at customer locations
- Conference Education: Training sessions at major medical conferences
- User Communities: Online forums and user groups for peer learning
-
Experience the Future of Spirometry
Transform your respiratory healthcare practice with the COSMED MicroQuark Spirometer’s revolutionary combination of Italian precision engineering and advanced AI cloud integration.
Contact Information:
📞 Phone: +1-800-426-7633
📧 Email: sales@cosmed.com
🌐 Website: www.cosmed.com
📍 Visit our global network of authorized dealers and service centers
Request a demonstration today and discover how the MicroQuark can revolutionize your respiratory diagnostic capabilities!
Conclusion
The COSMED MicroQuark Spirometer represents a paradigm shift in respiratory diagnostics, combining four decades of Italian engineering excellence with cutting-edge artificial intelligence and cloud connectivity. This revolutionary device transforms traditional spirometry from a simple diagnostic test into an intelligent, connected healthcare tool that provides immediate clinical insights and long-term health management capabilities.
The device’s advanced turbine technology eliminates many limitations of traditional spirometry systems while providing superior accuracy, reliability, and ease of use. The comprehensive integration with nine major AI cloud platforms creates unprecedented opportunities for clinical decision support, population health management, and respiratory research applications.
From primary care screening to specialized pulmonology practice, from occupational health to telemedicine applications, the MicroQuark adapts to diverse clinical needs while maintaining consistent, high-quality results. Its portability and durability make advanced spirometry accessible in settings where traditional equipment would be impractical, extending the reach of quality respiratory healthcare to underserved populations and remote locations.
The device’s exceptional value proposition combines superior technology with comprehensive support services, ensuring optimal performance and user satisfaction throughout its operational life. COSMED’s commitment to innovation ensures that the MicroQuark will continue to evolve with advancing technology and changing healthcare needs.
Healthcare providers choosing the COSMED MicroQuark Spirometer are investing in the future of respiratory diagnostics – a future where intelligent devices provide immediate insights, predictive analytics guide treatment decisions, and connected healthcare systems enable better outcomes for patients worldwide. The MicroQuark is not just a spirometer; it’s a gateway to the next generation of intelligent healthcare technology.
Experience the precision of Italian engineering. Embrace the power of artificial intelligence. Transform your respiratory healthcare practice with the COSMED MicroQuark Spirometer.