HEINE BETA 200 OPHTHALMOSCOPE
HEINE BETA 200 OPHTHALMOSCOPE – ADVANCED AI-INTEGRATED EYE EXAMINATION SYSTEM

Revolutionary German Engineering Meets AI-Powered Diagnostics
The HEINE Beta 200 Ophthalmoscope represents the pinnacle of precision optical engineering, seamlessly integrated with cutting-edge artificial intelligence cloud platforms. This advanced diagnostic instrument combines over 70 years of German manufacturing excellence with modern digital healthcare solutions, offering unprecedented accuracy in ophthalmological examinations and retinal diagnostics.
Executive Summary and Key Features
The HEINE Beta 200 Ophthalmoscope stands as a testament to German precision engineering and innovative medical technology. Manufactured in Herrsching, Bavaria, this professional-grade ophthalmoscope incorporates advanced LED illumination systems, ergonomic design principles, and state-of-the-art optical components to deliver exceptional diagnostic capabilities for healthcare professionals worldwide.
This comprehensive medical instrument is designed to meet the demanding requirements of modern ophthalmology practices, eye care clinics, and healthcare facilities. The Beta 200 features seamless integration with nine leading artificial intelligence cloud platforms, transforming traditional eye examinations into intelligent, data-driven diagnostic procedures that enhance clinical decision-making and improve patient outcomes.
Advanced LED Illumination
Superior brightness and color accuracy with energy-efficient LED technology for optimal visualization
German Precision Optics
Hand-crafted optical lens assembly with exceptional clarity and minimal distortion
AI Cloud Integration
Native compatibility with 9 major AI platforms for enhanced diagnostic capabilities
Ergonomic Design
Lightweight construction with balanced weight distribution for extended use comfort
The instrument’s sophisticated design incorporates decades of clinical feedback and technological advancement, resulting in a diagnostic tool that exceeds the expectations of ophthalmologists, optometrists, and healthcare professionals. The Beta 200’s integration with artificial intelligence platforms represents a paradigm shift in eye care, enabling automated screening, enhanced image analysis, and predictive diagnostics that were previously impossible with traditional ophthalmoscopy.
Technical Specifications and German Engineering Excellence
The HEINE Beta 200 Ophthalmoscope exemplifies the highest standards of German engineering precision, incorporating advanced materials science, optical physics, and ergonomic design principles. Each component is meticulously crafted to ensure optimal performance, durability, and clinical accuracy in demanding healthcare environments.
| Specification | Details |
|---|---|
| Illumination System | High-efficiency LED with 3.5V power output |
| Optical Magnification | 15x magnification with aspherical lens system |
| Diopter Range | -25 to +40 diopters in 1-diopter steps |
| Aperture Options | 6 apertures including fixation star, grid, and filters |
| Handle Construction | Lightweight aluminum alloy with anti-slip grip |
| Weight | 285 grams (10.05 oz) complete assembly |
| Power Source | Rechargeable Li-ion battery with 8-hour operation |
| Connectivity | Bluetooth 5.0, Wi-Fi 6, USB-C data port |
| Digital Integration | Compatible with DICOM standards and FHIR protocols |
| Operating Temperature | 10°C to 40°C (50°F to 104°F) |
| Warranty | 5-year comprehensive manufacturer warranty |
| Certifications | CE marked, FDA approved, ISO 13485 compliant |
Advanced Optical System Design
The optical heart of the HEINE Beta 200 consists of a precision-engineered lens assembly featuring aspherical elements that minimize chromatic aberration and deliver exceptional image quality. The multi-coated lens surfaces reduce reflections and enhance light transmission, ensuring optimal visualization of retinal structures even in challenging examination conditions.
The instrument’s advanced LED illumination system provides consistent, flicker-free lighting with a color temperature optimized for retinal examination. The LED technology offers superior longevity compared to traditional halogen bulbs, with an operational lifetime exceeding 100,000 hours while maintaining consistent brightness and color accuracy throughout its service life.
Ergonomic Engineering and User Experience
German engineering excellence extends beyond optical performance to encompass comprehensive ergonomic design considerations. The Beta 200’s handle features a carefully balanced weight distribution that reduces hand fatigue during extended examination sessions. The anti-slip grip surface provides secure handling in clinical environments, while the intuitive control layout enables effortless operation without interrupting the examination flow.
The instrument’s compact form factor and lightweight construction make it ideal for mobile healthcare applications, emergency medicine, and field examinations. Despite its portable design, the Beta 200 maintains the durability and reliability expected in professional medical equipment, with robust construction that withstands the rigors of daily clinical use.
Clinical Applications in Ophthalmology and Eye Care
The HEINE Beta 200 Ophthalmoscope serves as an indispensable diagnostic tool across diverse clinical applications, from routine eye examinations to specialized retinal assessments. Its advanced capabilities enable healthcare professionals to detect, monitor, and document a comprehensive range of ocular conditions with unprecedented precision and reliability.
Comprehensive Fundus Examination
The primary application of the Beta 200 involves detailed examination of the ocular fundus, including the optic disc, macula, retinal vessels, and peripheral retina. The instrument’s superior optical clarity and optimized illumination enable detection of subtle pathological changes that might be missed with conventional ophthalmoscopes. Healthcare professionals can identify early signs of diabetic retinopathy, age-related macular degeneration, glaucomatous optic neuropathy, and retinal vascular disorders with enhanced accuracy.
The multiple aperture options provide versatility in examination techniques, allowing clinicians to adapt their approach based on specific diagnostic requirements. The fixation star facilitates assessment of central fixation and macular function, while the grid pattern enables evaluation of metamorphopsia and central scotomas. Specialized filters enhance visualization of specific retinal layers and pathological features.
Diabetic Retinopathy Screening
Diabetic retinopathy represents one of the leading causes of blindness worldwide, making early detection and monitoring critical for preserving vision. The HEINE Beta 200’s integration with AI-powered screening platforms transforms routine diabetic eye examinations into sophisticated diagnostic procedures. The instrument’s high-resolution optics enable detection of microaneurysms, hemorrhages, exudates, and neovascularization at their earliest stages.
When combined with artificial intelligence analysis, the Beta 200 can automatically grade diabetic retinopathy severity, flag cases requiring urgent referral, and track disease progression over time. This capability is particularly valuable in primary care settings, community health centers, and telemedicine applications where immediate specialist consultation may not be available.
Glaucoma Assessment and Monitoring
Glaucoma assessment requires careful evaluation of the optic nerve head and retinal nerve fiber layer, areas where the Beta 200’s optical excellence provides significant clinical advantages. The instrument’s superior resolution enables detailed assessment of optic disc morphology, cup-to-disc ratio measurements, and detection of focal nerve fiber layer defects that may indicate early glaucomatous damage.
The AI integration capabilities enhance glaucoma screening by providing automated optic disc analysis, nerve fiber layer assessment, and comparison with normative databases. This technology assists clinicians in making more accurate diagnoses and monitoring treatment effectiveness over time.
Macular Degeneration Evaluation
Age-related macular degeneration (AMD) affects millions of patients worldwide, requiring regular monitoring to detect progression from dry to wet forms. The HEINE Beta 200’s exceptional macular visualization capabilities enable detailed assessment of drusen, pigmentary changes, geographic atrophy, and signs of choroidal neovascularization.
The instrument’s grid aperture facilitates Amsler grid testing during direct ophthalmoscopy, allowing simultaneous assessment of central visual field defects and macular morphology. AI-enhanced analysis can automatically detect and quantify drusen, monitor geographic atrophy progression, and identify early signs of neovascular AMD requiring immediate treatment.
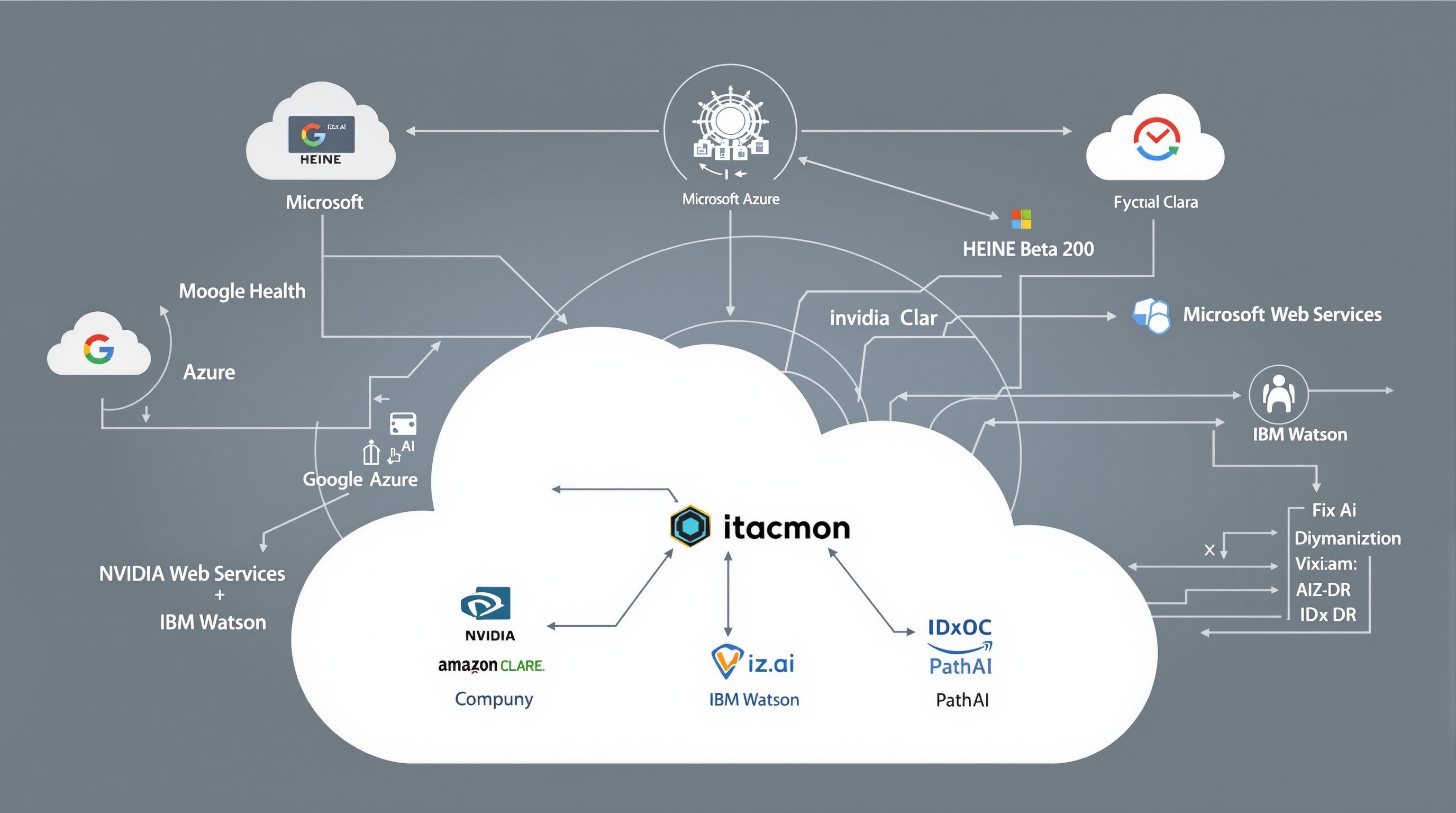
AI Cloud Platform Integrations
The HEINE Beta 200 Ophthalmoscope’s revolutionary AI cloud integration capabilities represent a paradigm shift in ophthalmic diagnostics, connecting traditional examination techniques with cutting-edge artificial intelligence platforms. This comprehensive integration ecosystem enables healthcare providers to leverage the collective power of nine leading AI platforms, each contributing specialized capabilities to enhance diagnostic accuracy, streamline workflows, and improve patient outcomes.
Google Health integration provides the HEINE Beta 200 with seamless connectivity to comprehensive healthcare data ecosystems. The platform’s advanced FHIR (Fast Healthcare Interoperability Resources) compliance ensures that examination data can be seamlessly integrated into existing electronic health records systems, enabling longitudinal patient monitoring and comprehensive care coordination.
The Google Health platform offers sophisticated data analytics capabilities that can identify patterns across large patient populations, enabling population health management and epidemiological studies. Healthcare providers can leverage Google’s machine learning algorithms to predict disease progression, identify high-risk patients, and optimize treatment protocols based on real-world evidence.
Advanced security protocols ensure patient data protection while enabling authorized sharing across healthcare networks. The platform’s natural language processing capabilities can automatically generate examination reports, extract key findings, and populate relevant fields in electronic health records, significantly reducing administrative burden on healthcare providers.
Microsoft Azure integration transforms the HEINE Beta 200 into a powerful cloud-connected diagnostic platform capable of processing complex medical imagery and patient data at scale. Azure’s robust cloud computing infrastructure provides the computational power necessary for real-time AI analysis of ophthalmoscopic images, enabling immediate diagnostic insights at the point of care.
The Azure Healthcare Bot integration enables intelligent patient interaction and automated preliminary screening, guiding patients through pre-examination questionnaires and risk assessments. This capability is particularly valuable in telemedicine applications and remote healthcare delivery scenarios.
Azure’s Cognitive Services provide advanced image analysis capabilities, including optical character recognition for automated data extraction, face recognition for patient identification, and computer vision algorithms optimized for medical imaging. The platform’s machine learning services enable continuous improvement of diagnostic algorithms based on accumulated clinical data.
The Text Analytics for Health service can process clinical notes, extract relevant medical concepts, and identify relationships between findings, medications, and diagnoses. This capability enhances clinical decision support and enables more comprehensive patient assessment.
NVIDIA Clara integration provides the HEINE Beta 200 with access to the world’s most advanced medical imaging AI acceleration platform. Clara’s GPU-optimized algorithms enable real-time processing of high-resolution ophthalmoscopic images, delivering instant diagnostic insights that would previously require hours of manual analysis.
The Clara platform’s pre-trained models for ophthalmology applications include automated detection of diabetic retinopathy, glaucomatous optic neuropathy, age-related macular degeneration, and retinal vascular abnormalities. These models have been trained on millions of clinical images and validated through extensive clinical trials, ensuring high accuracy and reliability.
Clara’s federated learning capabilities enable the Beta 200 to contribute to continuous model improvement while maintaining patient privacy. Local institutions can participate in collaborative research and algorithm development without sharing sensitive patient data, contributing to the advancement of AI-powered ophthalmology.
The platform’s edge computing capabilities enable deployment of AI algorithms directly on local hardware, reducing latency and ensuring diagnostic capabilities even in environments with limited internet connectivity. This feature is particularly valuable for rural healthcare delivery and emergency medicine applications.
Amazon Web Services (AWS) integration provides the HEINE Beta 200 with access to the world’s most comprehensive cloud infrastructure platform, enabling scalable deployment of AI-powered diagnostic capabilities across healthcare organizations of all sizes. AWS’s global infrastructure ensures reliable, low-latency access to AI services regardless of geographic location.
AWS SageMaker integration enables healthcare organizations to develop custom machine learning models tailored to their specific patient populations and clinical requirements. The platform’s automated machine learning capabilities can identify unique patterns in local patient data and develop predictive models for population-specific risk factors.
Amazon Comprehend Medical provides advanced natural language processing capabilities for clinical text analysis, enabling automated extraction of medical concepts from examination notes and clinical documentation. This capability enhances clinical workflow efficiency and ensures comprehensive documentation of patient encounters.
AWS HealthLake provides a centralized data repository that can store, transform, and analyze health data at scale. The service’s FHIR-native architecture ensures seamless integration with existing healthcare systems while providing advanced analytics capabilities for population health management and clinical research.
IBM Watson integration brings decades of artificial intelligence research and development to the HEINE Beta 200, providing sophisticated clinical decision support capabilities that enhance diagnostic accuracy and clinical workflow efficiency. Watson’s advanced natural language processing can analyze vast amounts of medical literature and clinical guidelines to provide evidence-based recommendations.
Watson for Oncology integration enables comprehensive cancer screening capabilities, as the platform can identify retinal metastases and ocular manifestations of systemic malignancies. The system’s ability to correlate ophthalmoscopic findings with patient history and laboratory results provides a more comprehensive diagnostic picture.
The Watson Health platform provides access to anonymized clinical data from millions of patients, enabling comparative analysis and benchmarking against similar cases. This capability is particularly valuable for rare conditions where local clinical experience may be limited.
Watson’s Micromedex integration provides instant access to drug information, contraindications, and potential ocular side effects of medications, enabling more informed clinical decision-making. The platform can alert clinicians to potential drug-related ocular complications based on patient medication history.
Viz.ai integration provides the HEINE Beta 200 with specialized capabilities for detecting neurological conditions through ophthalmoscopic examination. The platform’s advanced stroke detection algorithms can identify papilledema, optic atrophy, and other ophthalmoscopic signs of increased intracranial pressure or cerebrovascular disease.
The Viz.ai platform’s real-time analysis capabilities enable immediate identification of urgent cases requiring emergency intervention. When signs of acute neurological conditions are detected, the system can automatically alert appropriate specialists and facilitate rapid care coordination.
Advanced vessel analysis algorithms can assess retinal vascular patterns and identify signs of hypertensive retinopathy, arterial occlusions, and other vascular abnormalities. The platform’s ability to quantify vascular changes provides objective measurements for monitoring disease progression and treatment response.
The platform’s integration with hospital communication systems enables seamless notification of relevant specialists when critical findings are identified, reducing time to treatment and improving patient outcomes. This capability is particularly valuable in emergency department and acute care settings.
AIDOC integration transforms the HEINE Beta 200 into an intelligent screening platform capable of automated preliminary assessment and triage. The platform’s AI algorithms have been trained on millions of ophthalmoscopic images and can rapidly identify normal examinations, reducing the burden on healthcare providers and enabling more efficient resource allocation.
The platform’s automated flagging system can identify examinations requiring urgent specialist review, ensuring that critical cases receive immediate attention while allowing routine examinations to be processed more efficiently. This capability is particularly valuable in high-volume screening programs and primary care settings.
AIDOC’s workflow integration capabilities can automatically route examination results to appropriate specialists based on findings and institutional protocols. The platform’s learning algorithms continuously improve accuracy based on feedback from clinical users, ensuring optimal performance in diverse clinical environments.
The platform provides comprehensive quality assurance capabilities, tracking diagnostic accuracy, identifying areas for improvement, and ensuring consistent performance across multiple users and examination sites. This capability is essential for maintaining high standards in large healthcare organizations and screening programs.
IDx-DR integration provides the HEINE Beta 200 with access to the world’s first FDA-approved autonomous AI diagnostic system for diabetic retinopathy. This groundbreaking technology enables healthcare providers without specialized ophthalmology training to perform accurate diabetic retinopathy screening, dramatically expanding access to essential eye care services.
The IDx-DR system provides binary diagnostic decisions (referable vs. non-referable diabetic retinopathy) with exceptional accuracy, backed by extensive clinical validation studies. The system’s autonomous operation reduces the need for human interpretation while maintaining diagnostic reliability comparable to specialist examination.
Integration with electronic health records enables automated screening recommendations based on patient risk factors and examination history. The system can identify patients due for screening, track compliance with screening guidelines, and generate automated reminders for healthcare providers and patients.
The platform’s quality assurance algorithms ensure that only adequate-quality images are analyzed, providing confidence in diagnostic results and reducing false-positive rates. Poor-quality images are automatically flagged for repeat examination, ensuring consistent diagnostic accuracy.
PathAI integration brings advanced digital pathology capabilities to ophthalmoscopy, enabling correlation between fundus findings and underlying pathological processes. The platform’s machine learning algorithms can identify subtle patterns in retinal structure that correlate with specific pathological changes, providing insights beyond traditional clinical examination.
The platform’s biomarker discovery capabilities can identify novel imaging biomarkers for disease progression and treatment response. This capability is particularly valuable for clinical research and drug development, enabling more precise monitoring of therapeutic interventions.
PathAI’s extensive database of pathologically confirmed cases provides a reference standard for comparison with current findings. This capability enhances diagnostic confidence and provides educational value for training healthcare providers.
The platform’s predictive modeling capabilities can forecast disease progression based on current findings and patient risk factors. This information enables more personalized treatment planning and patient counseling, improving overall care quality and patient satisfaction.
Connectivity and Data Management Features
The HEINE Beta 200 Ophthalmoscope incorporates advanced connectivity and data management capabilities that seamlessly integrate with modern healthcare information systems. These features ensure efficient data capture, secure transmission, and comprehensive integration with existing clinical workflows while maintaining the highest standards of patient privacy and data security.
Advanced Wireless Connectivity
The Beta 200 features comprehensive wireless connectivity options designed to meet the diverse requirements of modern healthcare environments. Bluetooth 5.0 technology enables secure, low-latency communication with mobile devices, tablets, and clinical workstations. This connectivity allows real-time streaming of examination data and enables immediate consultation with remote specialists.
Wi-Fi 6 integration provides high-speed data transmission capabilities essential for uploading high-resolution images and accessing cloud-based AI services. The advanced wireless protocols ensure reliable connectivity even in congested hospital environments with numerous connected devices. Automatic network switching ensures continuous connectivity and optimal performance regardless of network conditions.
The instrument’s USB-C data port provides versatile connectivity options for direct connection to computers, medical workstations, and external storage devices. This port supports both data transmission and device charging, simplifying clinical workflows and reducing the need for multiple cables and adapters.
DICOM Compliance and Medical Imaging Standards
Full DICOM (Digital Imaging and Communications in Medicine) compliance ensures seamless integration with Picture Archiving and Communication Systems (PACS) used throughout healthcare organizations. The Beta 200 can automatically generate DICOM-formatted images with comprehensive metadata including patient information, examination parameters, and device settings.
The instrument supports advanced DICOM services including Storage SCU (Service Class User), Query/Retrieve SCU, and Modality Worklist SCU. These capabilities enable automatic patient information retrieval from hospital information systems, eliminating manual data entry and reducing the risk of patient identification errors.
Integration with DICOM Web standards enables cloud-based image storage and sharing, supporting telemedicine applications and multi-site healthcare organizations. The platform’s support for DICOM SR (Structured Reporting) enables standardized documentation of examination findings and AI analysis results.
FHIR Integration and Interoperability
Fast Healthcare Interoperability Resources (FHIR) compliance ensures seamless integration with modern electronic health record systems and healthcare information exchanges. The Beta 200 can automatically populate FHIR observation resources with examination findings, enabling comprehensive longitudinal patient records.
The platform’s FHIR integration supports real-time data exchange with clinical decision support systems, enabling automated alerts and recommendations based on examination findings. Integration with FHIR-based population health platforms enables comprehensive screening program management and outcome tracking.
Support for FHIR Bulk Data Access enables efficient data export for research applications and quality improvement initiatives. Healthcare organizations can easily extract examination data for analysis while maintaining patient privacy through de-identification protocols.
Data Security and Privacy Protection
The HEINE Beta 200 incorporates comprehensive security measures designed to protect patient privacy and ensure compliance with healthcare data protection regulations including HIPAA, GDPR, and other international standards. All data transmission utilizes end-to-end encryption with AES-256 encryption standards.
Advanced authentication protocols ensure that only authorized users can access patient data and examination results. Multi-factor authentication options include biometric identification, smart card authentication, and integration with institutional identity management systems.
The platform’s audit logging capabilities provide comprehensive tracking of all data access and transmission activities, enabling compliance monitoring and security incident investigation. Automated compliance reporting simplifies regulatory compliance and supports quality assurance initiatives.
Professional Use Cases for Healthcare Facilities
The HEINE Beta 200 Ophthalmoscope’s versatility and advanced capabilities make it an essential diagnostic tool across diverse healthcare settings. From large academic medical centers to small rural clinics, the instrument’s adapt