How to Clean and Sterilize Reusable Surgical Tools: A Comprehensive Guide to Infection Prevention
Ensuring the sterility of surgical instruments and other medical equipment is paramount to patient safety in hospitals, ambulatory surgical centers (ASCs), and other healthcare facilities. Proper cleaning and sterilization protocols prevent cross-contamination, reduce the risk of healthcare-associated infections (HAIs), and maintain equipment integrity while complying with medical device standards and regulations.
Understanding the Fundamentals: Cleaning vs. Disinfection vs. Sterilization
Before delving into the specific procedures, it’s crucial to understand the distinctions between cleaning, disinfection, and sterilization, as these terms are often used interchangeably but have different meanings and applications.
Cleaning is the first and most critical step in the decontamination process. It involves removing any visible dirt, debris, blood, tissue, and organic matter from medical equipment using water, detergents, or enzymatic products. While cleaning removes physical contaminants from instruments, it does not eliminate all microorganisms present on their surface.
Disinfection goes beyond cleaning by destroying most pathogenic microorganisms on inanimate objects through chemical agents or physical methods such as UV light. However, it does not necessarily kill all microbial life forms, such as bacterial spores. Medical facilities typically employ varying levels of disinfection—low, intermediate, or high—depending on the microorganisms present and the intended use of the items being disinfected.
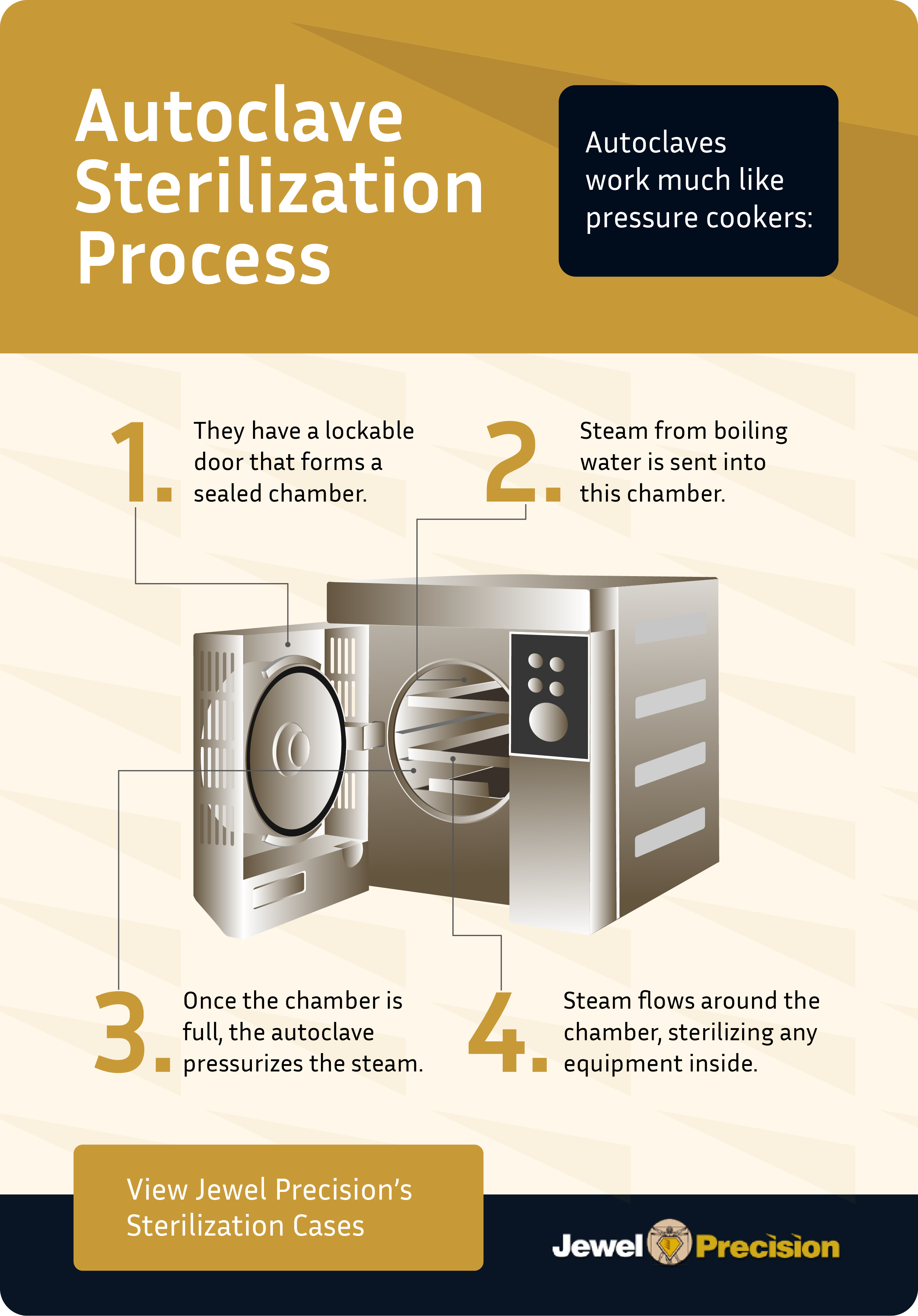
Sterilization represents the most rigorous form of decontamination, designed to destroy all forms of bacterial life present on surgical instruments, including bacterial spores. Common sterilization methods include steam under pressure (autoclaving), ethylene oxide (EtO) gas, hydrogen peroxide plasma, and dry heat. Steam sterilization is often the preferred method for sterile processing departments (SPDs) due to its efficiency, cost-effectiveness, and ability to penetrate fabrics and complex instruments.

Regulatory Standards and Guidelines
Several organizations establish strict requirements for cleaning surgical instruments to maintain the highest levels of hygiene and safety in healthcare settings:
The Association for the Advancement of Medical Instrumentation (AAMI), in partnership with the American National Standards Institute, developed ST79, a comprehensive guide outlining best practices for healthcare product sterilization. This standard covers sterilization equipment, process validation, quality control, and staff training to ensure consistent and effective sterilization procedures.
The Centers for Disease Control and Prevention (CDC) sets infection control guidelines, including those for cleaning and sterilizing surgical instruments and other medical devices CDC Guidelines. These guidelines include requirements for pre-cleaning instruments, recommendations for choosing appropriate cleaning agents, and procedures for disinfecting and sterilizing equipment according to risk levels.
The World Health Organization (WHO) offers global guidelines for infection prevention, including medical device sterilization, emphasizing point-of-use (POU) cleaning, consistent decontamination, and appropriate staff training.

The Critical Importance of Point-of-Use Cleaning
Point-of-use cleaning represents the initial step of the surgical instrument decontamination process and takes place immediately after use. This crucial phase involves removing gross contaminants such as blood and tissue at the site of use before instruments are transported to the SPD for more thorough cleaning and sterilization.
POU cleaning prevents biofilm formation—clusters of microorganisms that stick to non-biological surfaces—by removing organic material immediately. Biofilm can harbor and protect bacteria, making subsequent cleaning and sterilization more challenging. Additionally, early removal of contaminants reduces the microbial load on instruments, enhancing the efficacy of later disinfection and sterilization processes.

During POU cleaning, it’s best to wipe instruments down with a sterile, damp cloth or sponge and use a pretreatment spray, foam, or gel to keep organic matter moist. For heavily soiled instruments, soaking them in a container filled with an enzymatic detergent solution can help break up organic matter, making them easier to clean during subsequent processing stages.
Comprehensive 12-Step Cleaning and Sterilization Process
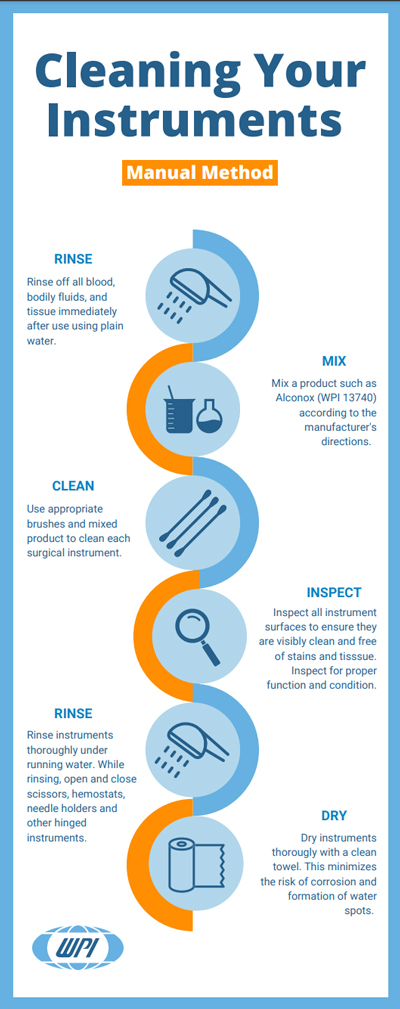
Step 1: Point-of-Use Cleaning
Initiate cleaning immediately after surgical procedures to prevent blood, bodily fluids, and other contaminants from drying on instruments and forming biofilm. Use sterile, damp cloths or sponges with pretreatment sprays, foams, or gels to maintain organic matter moisture.
Step 2: Safe Transportation
Transport instruments from the surgical site to the SPD in leak-proof, puncture-resistant containers clearly labeled with biohazard symbols to minimize contamination or damage risks.
Step 3: Manual Cleaning
Perform thorough manual cleaning by physically scrubbing surgical instruments with soft-bristled brushes, lint-free cloths, and appropriate detergents to remove all visible soil. Pay particular attention to joints, crevices, lumens, and other intricate parts to ensure no remaining debris or residue. Complete this step by rinsing with distilled or deionized water to remove residual cleaning agents.

Step 4: Mechanical Cleaning
Utilize mechanical cleaning methods, typically performed in ultrasonic cleaners or washer-disinfectors, to remove remaining debris using high-frequency sound waves or automated washing cycles. Properly load instruments to ensure full exposure to the cleaning process and select appropriate cycle settings based on manufacturer Instructions for Use (IFU).

Step 5: Ultrasonic Cleaning Details
Ultrasonic cleaning utilizes high-frequency sonic waves that create tiny bubbles on instrument surfaces through a process called cavitation. These bubbles eventually implode, helping dislodge soil from device surfaces. Use low-foaming enzymatic cleaners in ultrasonic cleaners, ensuring foam doesn’t interfere with the cavitation process. After ultrasonic cleaning, thoroughly rinse instruments with deionized or softened water.

Step 6: Washer-Disinfector Processing
Washer-disinfectors rely on spray arm technology with pressurized water to clean surgical instruments. The load experiences specific water temperatures, chemical concentrations, and flow rates. The thermal rinse phase provides disinfection levels, with optional drying phases to reduce manual drying requirements.
Step 7: Sterilization
Execute the most critical step by eliminating all forms of microbial life, including bacterial spores, biofilm, and protozoa. Choose appropriate sterilization methods—ethylene oxide gas, hydrogen peroxide plasma, dry heat, or steam sterilization—following manufacturer IFUs.
Step 8: Post-Sterilization Rinsing
If ethylene oxide was used for sterilization, rinse instruments afterward using distilled or deionized water to remove any residual EtO, which can be toxic to patients and staff.
Step 9: Thorough Drying
Allow steam-sterilized or EtO-processed instruments time to cool or aerate, then thoroughly dry them using lint-free towels or compressed air. This step maintains instrument integrity, especially for metal instruments that can rust and corrode if packaged while wet.
Step 10: Lubrication
Apply medical-grade, water-soluble lubricant sparingly to hinges and joints of surgical instruments with moving parts to maintain functionality and prolong lifespan, following manufacturer IFUs.
Step 11: Comprehensive Inspection
Inspect each instrument for cleanliness, functionality, and integrity before packaging using magnification to identify cracks, chips, or other damage. Ensure instruments with moving parts operate smoothly and correctly, removing and reprocessing any instruments that don’t meet cleanliness or functionality standards.
Step 12: Proper Packaging and Storage
Package surgical instruments using appropriate materials such as sterilization wraps, pouches, or containers, sealing tightly to prevent contamination. Label packages with sterilization dates, contents, and relevant batch numbers for traceability. Store sterilized instruments in clean, dry, temperature-controlled environments on shelves rather than floors, away from direct sunlight.

Advanced Cleaning Technologies
Ultrasonic Cleaning Systems
Ultrasonic cleaners work through cavitation, where high-frequency sonic waves create microscopic bubbles that implode on instrument surfaces, effectively removing debris from hard-to-reach areas like crevices, hinges, and lumens. These systems are particularly effective for complex instruments with intricate geometries.

Automated Washer-Disinfectors
These systems provide consistent and efficient cleaning through controlled parameters including time, temperature, chemistry, and mechanical action. They offer improved productivity, reduced staff exposure to contaminated materials, and standardized cleaning results.
Common Points of Failure and Prevention
Several potential failure points can compromise the effectiveness of surgical instrument cleaning and sterilization:
Delayed Cleaning: Allowing bioburden to dry on instruments creates biofilm that shields bacteria from cleaning and sterilization processes. Immediate POU cleaning is essential.
Inadequate Disassembly: Instruments not properly disassembled prior to cleaning can harbor residual debris in joints and hidden areas, compromising sterilization effectiveness.
Incorrect Cleaning Solutions: Using inappropriate cleaning agents can result in ineffective bioburden removal or instrument damage. Always use manufacturer-recommended solutions.
Insufficient Staff Training: Inadequately trained technicians may compromise the entire reprocessing chain. Continuous education and competency assessments are essential.
Equipment Overloading: Overcrowding cleaning and sterilization equipment prevents proper exposure to cleaning agents and sterilizing conditions.

Quality Control and Monitoring
Effective quality control measures ensure consistent sterilization standards:
Biological Indicators: Use spore tests to directly monitor sterilization process lethality. These indicators contain the most resistant microorganisms and provide the most reliable assessment of sterilization effectiveness.
Chemical Indicators: Employ these convenient, inexpensive indicators to show exposure to sterilization processes, though they should supplement, not replace, biological indicators.
Mechanical Monitoring: Regularly assess cycle parameters including time, temperature, and pressure through record charts and computer printouts.
Cleaning Verification: Implement ATP or protein detection systems to identify cleaning process gaps beyond visual inspection.
Storage and Shelf Life Management
Proper storage maintains instrument sterility until use. Implement event-related shelf-life practices recognizing that products remain sterile until contaminating events occur, such as package tears or moisture exposure. Store sterile supplies with adequate clearances: 8-10 inches from floors, 5 inches from ceilings, and 2 inches from walls to allow proper air circulation and comply with fire codes.
Best Practices for Optimal Results
Establish comprehensive protocols that include immediate post-use cleaning, strict adherence to manufacturer IFUs, maintaining separate contaminated and clean areas, requiring appropriate PPE for all SPD technicians, implementing rigorous quality control measures, and providing ongoing staff training and competency assessments.

Conclusion
Proper cleaning and sterilization of reusable surgical instruments represents a critical component of infection prevention in healthcare settings. By following established guidelines, implementing comprehensive quality control measures, and maintaining rigorous protocols, healthcare facilities can ensure patient safety while preserving instrument integrity and functionality. The investment in proper reprocessing procedures significantly outweighs the potential costs and risks associated with healthcare-associated infections and compromised patient outcomes.
Success in surgical instrument reprocessing requires commitment to evidence-based practices, continuous staff education, regular equipment maintenance, and adherence to established standards from organizations like the CDC, AAMI, and WHO. Through diligent attention to these principles, healthcare facilities can maintain the highest standards of patient care while supporting positive clinical outcomes.
Sources: CDC Sterilization Guidelines, STERIS Cleaning Guide, Consolidated Sterilizer Systems