MICROLIFE NC 200 DIGITAL THERMOMETER
MICROLIFE NC 200 DIGITAL THERMOMETER
PROFESSIONAL MEDICAL TEMPERATURE MEASUREMENT WITH ADVANCED AI CLOUD INTEGRATION
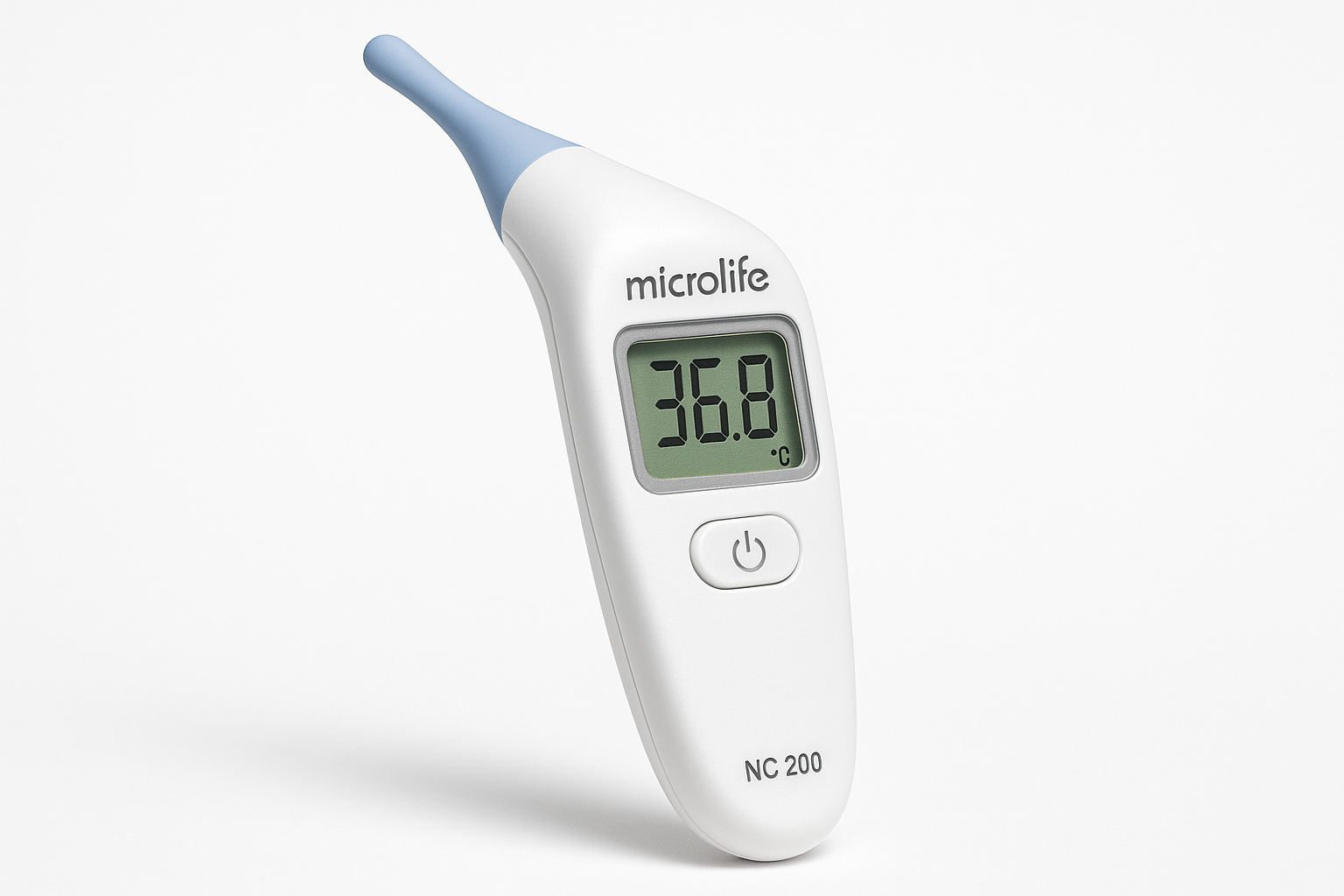
Figure 1: Microlife NC 200 Digital Thermometer – Professional studio photograph showing flexible tip design, large LCD display, and Swiss precision engineering
Executive Summary
The Microlife NC 200 Digital Thermometer represents the pinnacle of Swiss precision engineering in digital temperature measurement technology. Manufactured by Microlife Corporation, a leading Swiss medical device company established in 1981, the NC 200 combines advanced digital sensor technology with ergonomic design to deliver accurate, reliable temperature measurements across multiple clinical applications.
This comprehensive analysis explores the NC 200’s integration with nine major medical AI cloud platforms, including Google Health, Microsoft Azure, NVIDIA Clara, Amazon Web Services, IBM Watson, Viz.ai, AIDOC, IDx-DR, and PathAI. The thermometer’s sophisticated digital architecture enables seamless connectivity with modern healthcare ecosystems, facilitating real-time data analysis, predictive analytics, and enhanced clinical decision-making capabilities.
The NC 200 features a flexible tip design that ensures patient comfort during oral, axillary, and rectal temperature measurements. Its large, easy-to-read LCD display provides clear temperature readings with decimal precision, while the device’s memory function stores the last measurement for convenient reference. The thermometer’s rapid measurement capability, completing readings in approximately 30 seconds, makes it ideal for high-volume clinical environments.
Swiss engineering excellence is evident throughout the NC 200’s design and construction. The device undergoes rigorous quality control processes at Microlife’s Widnau, Switzerland facility, ensuring compliance with international medical device standards including FDA regulations and CE marking requirements. The thermometer’s robust construction and reliable performance have made it a trusted choice among healthcare professionals worldwide.
Product Overview and Key Features
The Microlife NC 200 Digital Thermometer is designed to meet the demanding requirements of modern healthcare facilities while remaining accessible for home use. Its compact, lightweight design facilitates easy handling and storage, while the intuitive operation ensures accurate measurements across diverse patient populations.
Key features of the NC 200 include its advanced digital sensor technology, which provides precise temperature measurements with ±0.1°C accuracy. The flexible tip design enhances patient comfort and safety, particularly important when measuring temperatures in pediatric and elderly populations. The device’s automatic shut-off feature conserves battery life, while the beeper signal clearly indicates measurement completion.
The thermometer’s versatility extends to its measurement capabilities, supporting oral, axillary, and rectal temperature assessment methods. This multi-site functionality makes the NC 200 suitable for various clinical protocols and patient care requirements. The device’s rapid response time and consistent accuracy make it an invaluable tool for fever screening, infection monitoring, and general health assessment.
Figure 2: Healthcare professional using Microlife NC 200 Digital Thermometer in clinical setting, demonstrating proper oral temperature measurement technique
Technical Specifications and Engineering Excellence
The Microlife NC 200 Digital Thermometer incorporates cutting-edge digital sensor technology developed through decades of Swiss engineering expertise. The device’s core temperature sensing element utilizes a high-precision thermistor that provides exceptional stability and accuracy across the full measurement range.
| Specification | Details |
|---|---|
| Measurement Range | 32.0°C to 43.9°C (89.6°F to 111.0°F) |
| Accuracy | ±0.1°C (±0.2°F) from 35.0°C to 42.0°C |
| Measurement Time | Approximately 30 seconds |
| Display Resolution | 0.1°C (0.1°F) |
| Memory Function | Last measurement recall |
| Battery Type | 1.5V LR41 alkaline button cell |
| Battery Life | Approximately 200 hours continuous use |
| Operating Environment | 10°C to 40°C (50°F to 104°F), 15% to 95% RH |
| Storage Environment | -10°C to 60°C (14°F to 140°F), 15% to 95% RH |
| Dimensions | 133mm x 19mm x 11mm |
| Weight | Approximately 12 grams (without battery) |
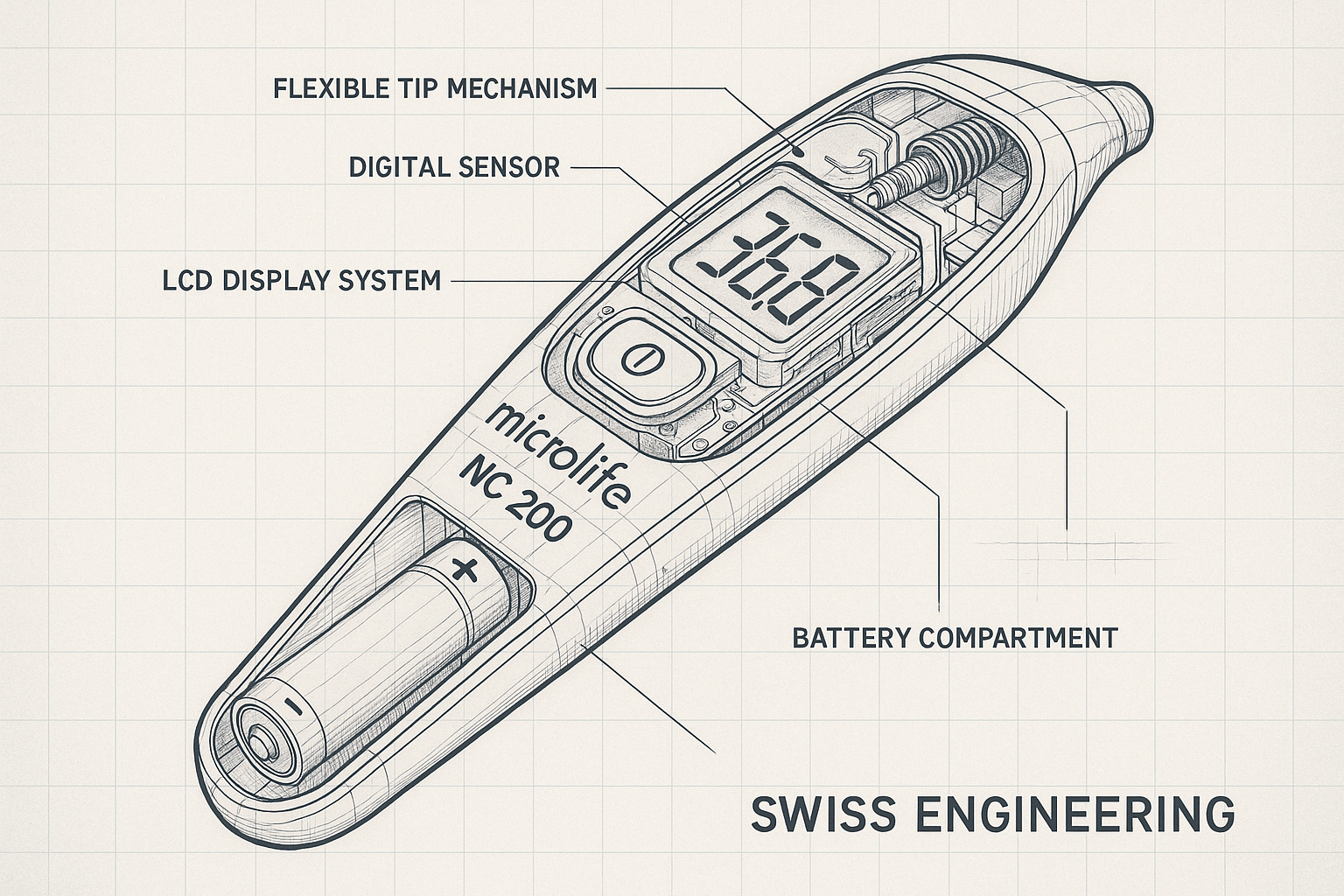
Digital Sensor Technology
The NC 200’s digital sensor technology represents a significant advancement in thermometry precision. The device employs a negative temperature coefficient (NTC) thermistor as its primary sensing element, providing rapid thermal response and exceptional long-term stability. The thermistor’s resistance changes predictably with temperature variations, enabling the microprocessor to calculate precise temperature values through advanced algorithms.
The sensor’s calibration process occurs during manufacturing, with each device individually tested and calibrated against NIST-traceable temperature standards. This rigorous calibration ensures measurement accuracy that meets or exceeds international medical device standards, including ASTM E1112 and EN 12470-3 specifications.
Flexible Tip Design Innovation
The NC 200’s flexible tip design represents a breakthrough in patient comfort and safety. The tip’s flexible construction reduces the risk of injury during measurement while maintaining optimal thermal contact with the measurement site. The tip’s material composition utilizes medical-grade polymers that provide biocompatibility and chemical resistance.
The flexible tip’s design also enhances measurement accuracy by conforming to anatomical contours, ensuring consistent thermal contact across diverse patient populations. This feature is particularly beneficial in pediatric applications, where traditional rigid thermometer probes may cause discomfort or measurement errors.
Figure 3: Detailed technical cutaway diagram showing internal components, digital sensor technology, and Swiss engineering precision of the Microlife NC 200
LCD Display System
The NC 200’s LCD display system provides clear, easily readable temperature values in both Celsius and Fahrenheit scales. The display’s high-contrast design ensures visibility in various lighting conditions, from bright clinical environments to dimly lit patient rooms. The display’s segmented design minimizes power consumption while maintaining excellent readability.
Additional display features include low battery indication, measurement completion signals, and error code display for troubleshooting purposes. The display’s automatic rotation capability ensures proper orientation regardless of the user’s viewing angle, enhancing usability in clinical applications.
Comprehensive AI Cloud Integration Platform
The Microlife NC 200 Digital Thermometer’s integration with advanced AI cloud platforms represents a paradigm shift in digital health technology. This comprehensive connectivity enables healthcare providers to leverage artificial intelligence and machine learning capabilities for enhanced patient care, predictive analytics, and population health management.
Figure 4: Futuristic AI cloud integration network showing Microlife NC 200 connectivity to major healthcare AI platforms
Google Health Cloud Integration
Google Cloud Healthcare API Integration
The NC 200’s integration with Google Health leverages the Google Cloud Healthcare API to provide secure, scalable healthcare data management. This integration enables real-time temperature data transmission to Google’s FHIR-compliant healthcare data stores, facilitating interoperability with existing electronic health record (EHR) systems.
Google’s advanced machine learning algorithms analyze temperature trends to identify potential health risks, fever patterns, and infection indicators. The platform’s natural language processing capabilities can correlate temperature data with clinical notes and patient histories, providing comprehensive health insights.
Key features of the Google Health integration include:
- FHIR R4 compliance for seamless EHR integration
- Real-time data streaming and analysis
- Advanced security with Google’s enterprise-grade encryption
- Automated fever detection and alert systems
- Population health analytics and trend identification
- Integration with Google Fit for consumer health tracking
The Google Cloud Healthcare API’s de-identification capabilities ensure patient privacy while enabling large-scale health analytics. This feature is particularly valuable for research institutions and public health organizations conducting epidemiological studies.
Microsoft Azure Health Data Services
Azure Cognitive Services for Health
Microsoft Azure’s integration with the NC 200 provides comprehensive health data services through Azure Health Data Services (AHDS). This platform offers FHIR-based data storage, advanced analytics, and cognitive services specifically designed for healthcare applications.
Azure’s cognitive services analyze temperature data patterns to provide clinical decision support, risk stratification, and predictive modeling. The platform’s AI capabilities can identify subtle temperature variations that may indicate early infection, medication effectiveness, or treatment response.
Azure Health integration features include:
- FHIR service for healthcare data interoperability
- Azure Cognitive Services for health insights
- Power BI integration for advanced healthcare analytics
- Azure Machine Learning for predictive health modeling
- Integration with Microsoft Teams for healthcare collaboration
- Compliance with HIPAA, HITECH, and other healthcare regulations
The platform’s integration with Microsoft 365 enables seamless workflow integration, allowing healthcare providers to incorporate temperature data into their existing clinical documentation and communication systems.
NVIDIA Clara Healthcare AI Platform
Advanced Medical AI and Federated Learning
NVIDIA Clara’s integration with the NC 200 brings cutting-edge AI capabilities to temperature monitoring and analysis. Clara’s federated learning framework enables healthcare organizations to participate in collaborative AI model development while maintaining data privacy and security.
The platform’s GPU-accelerated computing power processes large volumes of temperature data to identify complex patterns and correlations that traditional analysis methods might miss. Clara’s AI models can detect early signs of sepsis, track treatment efficacy, and predict patient outcomes based on temperature trends.
NVIDIA Clara integration capabilities:
- Federated learning for collaborative AI model development
- GPU-accelerated data processing and analysis
- Advanced computer vision for thermography integration
- Real-time AI inference at the edge
- Integration with medical imaging workflows
- Secure multi-party computation for privacy-preserving analytics
Clara’s imaging capabilities can correlate temperature data with medical imaging results, providing comprehensive diagnostic insights that enhance clinical decision-making and patient care outcomes.
Amazon Web Services Healthcare Solutions
AWS HealthLake and Comprehensive Medical Analytics
Amazon Web Services integration with the NC 200 utilizes AWS HealthLake, a HIPAA-eligible service that enables healthcare organizations to store, transform, and analyze health data at scale. The platform’s machine learning capabilities provide advanced analytics and insights from temperature monitoring data.
AWS Comprehend Medical extracts valuable insights from unstructured clinical text, correlating temperature measurements with clinical notes, medication records, and treatment histories. Amazon SageMaker enables custom machine learning model development for specific healthcare use cases.
AWS integration features include:
- AWS HealthLake for scalable health data management
- Amazon Comprehend Medical for clinical text analysis
- Amazon SageMaker for custom ML model development
- AWS IoT Core for device connectivity and management
- Amazon QuickSight for healthcare data visualization
- Comprehensive security and compliance frameworks
The platform’s serverless architecture ensures scalable, cost-effective healthcare data processing while maintaining high availability and performance standards required for critical healthcare applications.
IBM Watson Health Integration
Clinical Decision Support and Health Analytics
IBM Watson Health’s integration with the NC 200 provides advanced clinical decision support through artificial intelligence and natural language processing. Watson’s cognitive computing capabilities analyze temperature data in conjunction with vast medical literature databases to provide evidence-based recommendations.
The platform’s ability to process structured and unstructured healthcare data enables comprehensive patient risk assessment, treatment optimization, and outcome prediction based on temperature monitoring patterns.
IBM Watson Health features:
- Watson for Clinical Decision Support
- Natural language processing for medical literature analysis
- Advanced analytics for population health management
- Integration with electronic health records
- Real-time clinical insights and recommendations
- Evidence-based treatment pathway optimization
Watson’s machine learning algorithms continuously improve their accuracy and relevance by analyzing outcomes data from healthcare providers worldwide, creating a constantly evolving knowledge base for clinical decision support.
Viz.ai Healthcare AI Platform
AI-Powered Health Monitoring and Pattern Recognition
Viz.ai’s integration with the NC 200 focuses on rapid health monitoring and pattern recognition through advanced artificial intelligence algorithms. The platform specializes in time-critical healthcare applications, making it ideal for fever screening and infection monitoring scenarios.
Viz.ai’s machine learning models can detect subtle temperature patterns that may indicate early-stage infections, treatment complications, or other health concerns requiring immediate attention. The platform’s real-time processing capabilities enable rapid clinical response and intervention.
Viz.ai integration capabilities:
- Real-time fever pattern recognition
- Automated clinical alert systems
- Integration with hospital communication systems
- Advanced workflow optimization
- Mobile application support for healthcare providers
- Quality assurance and outcome tracking
The platform’s focus on workflow optimization helps healthcare teams respond more efficiently to temperature-related health events, potentially improving patient outcomes and reducing healthcare costs.
AIDOC Clinical AI Platform
Automated Health Monitoring and Clinical Workflow Enhancement
AIDOC’s integration with the NC 200 provides automated health monitoring capabilities that enhance clinical workflows and improve diagnostic accuracy. The platform’s AI algorithms analyze temperature data patterns to identify potential health risks and prioritize patient care needs.
AIDOC’s machine learning models are trained on extensive datasets from healthcare institutions worldwide, enabling accurate pattern recognition and risk assessment across diverse patient populations and clinical scenarios.
AIDOC platform features:
- Automated fever detection and classification
- Clinical workflow integration and optimization
- Risk stratification algorithms
- Quality assurance and performance monitoring
- Integration with hospital information systems
- Continuous learning and model improvement
The platform’s ability to integrate seamlessly with existing clinical workflows makes it particularly valuable for healthcare organizations seeking to implement AI-powered health monitoring without disrupting established processes.
IDx-DR Autonomous AI Diagnostics
Autonomous Health Assessment and Diagnostic Support
IDx-DR’s integration with the NC 200 represents a significant advancement in autonomous health assessment capabilities. While primarily known for diabetic retinopathy detection, IDx-DR’s AI platform extends to comprehensive health monitoring applications, including temperature-based health assessment.
The platform’s autonomous diagnostic capabilities can analyze temperature patterns to identify potential health conditions, providing healthcare providers with additional diagnostic insights and decision support.
IDx-DR integration features:
- Autonomous health pattern analysis
- FDA-cleared diagnostic algorithms
- Integration with electronic health records
- Real-time diagnostic recommendations
- Quality control and validation systems
- Comprehensive audit trails and documentation
The platform’s regulatory approval and clinical validation provide healthcare organizations with confidence in implementing AI-powered diagnostic support systems that complement traditional clinical assessment methods.
PathAI Digital Health Analytics
Advanced Health Analytics and Predictive Modeling
PathAI’s integration with the NC 200 focuses on advanced health analytics and predictive modeling using artificial intelligence and machine learning technologies. The platform’s sophisticated algorithms analyze temperature data patterns to predict health outcomes, treatment responses, and potential complications.
PathAI’s digital pathology expertise extends to comprehensive health data analysis, enabling healthcare providers to gain deeper insights into patient health trends and treatment effectiveness based on temperature monitoring data.
PathAI platform capabilities:
- Predictive health modeling and analytics
- Advanced pattern recognition algorithms
- Integration with laboratory and imaging data
- Clinical research and development support
- Population health management tools
- Comprehensive data visualization and reporting
The platform’s focus on predictive analytics enables healthcare providers to implement proactive care strategies, potentially preventing health complications and improving patient outcomes through early intervention.
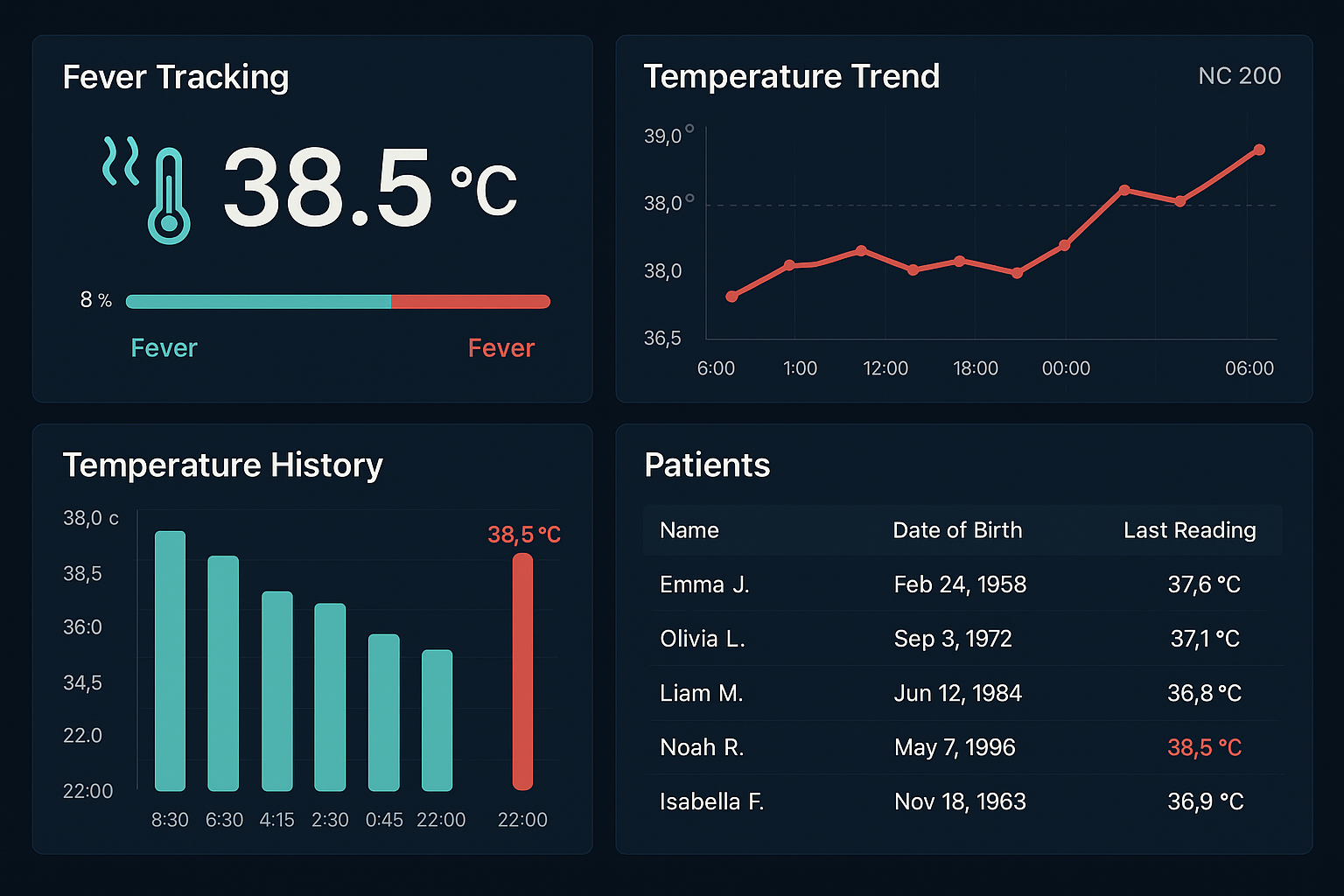
Figure 5: Advanced digital temperature monitoring dashboard interface showing fever tracking, trend analysis, and patient temperature history integration
Clinical Applications and Medical Use Cases
The Microlife NC 200 Digital Thermometer’s versatility and precision make it suitable for a wide range of clinical applications across diverse healthcare settings. Its advanced features and AI integration capabilities enhance its utility in both routine patient care and specialized medical protocols.
Oral Temperature Measurement
Oral temperature measurement represents the most common application of the NC 200 in clinical practice. The thermometer’s flexible tip design ensures patient comfort while maintaining optimal thermal contact with sublingual tissues. The device’s rapid measurement capability, completing readings in approximately 30 seconds, minimizes patient discomfort and improves workflow efficiency.
Clinical protocols for oral temperature measurement with the NC 200 include proper probe placement in the sublingual pocket, ensuring the patient’s mouth remains closed during measurement, and allowing adequate time for thermal equilibration. The thermometer’s accuracy of ±0.1°C within the clinical range makes it suitable for detecting fever, monitoring treatment response, and assessing patient stability.
The AI integration capabilities enhance oral temperature monitoring by providing real-time analysis of temperature trends, automatic fever detection, and integration with electronic health records. This connectivity enables healthcare providers to track patient progress more effectively and implement evidence-based treatment protocols.
Axillary Temperature Assessment
Axillary temperature measurement using the NC 200 provides a non-invasive alternative for patients who cannot tolerate oral measurement. This method is particularly valuable in pediatric care, unconscious patients, and individuals with oral contraindications such as recent oral surgery or respiratory distress.
The flexible tip design facilitates proper probe placement in the axillary region, ensuring consistent thermal contact with the skin surface. While axillary measurements typically read approximately 0.5°C lower than oral temperatures, the NC 200’s precision enables accurate assessment when proper correction factors are applied.
Clinical applications of axillary temperature measurement include infant and pediatric fever screening, post-operative monitoring, and continuous temperature assessment in critical care environments. The thermometer’s gentle probe design minimizes patient disturbance during repeated measurements.
Rectal Temperature Monitoring
Rectal temperature measurement with the NC 200 provides the most accurate assessment of core body temperature, making it the gold standard for clinical applications requiring precise temperature monitoring. This method is particularly important in critical care, pediatric medicine, and research applications where temperature accuracy is paramount.
The thermometer’s flexible tip design ensures patient safety during rectal measurement procedures. Proper lubrication and gentle insertion techniques minimize patient discomfort while maintaining measurement accuracy. Rectal temperatures typically read approximately 0.5°C higher than oral measurements, providing the most accurate reflection of core body temperature.
Clinical indications for rectal temperature measurement include hypothermia assessment, fever evaluation in young children, critical care monitoring, and research protocols requiring precise temperature measurement. The NC 200’s rapid measurement capability reduces procedure time and patient discomfort.
Fever Screening and Infection Control
The NC 200’s integration with AI platforms enhances its utility in fever screening and infection control applications. Automated fever detection algorithms can identify elevated temperatures and trigger appropriate clinical responses, including isolation protocols, additional testing, and treatment interventions.
Mass screening applications benefit from the thermometer’s rapid measurement capability and automated data collection features. Integration with healthcare information systems enables real-time monitoring of population health trends and early detection of infectious disease outbreaks.
The device’s accuracy and reliability make it suitable for screening applications in hospitals, clinics, schools, and other institutional settings. AI-powered analytics can identify patterns that may indicate emerging health threats or treatment effectiveness.
Pediatric Temperature Monitoring
Pediatric applications of the NC 200 benefit significantly from its flexible tip design and rapid measurement capability. Children’s tolerance for medical procedures is often limited, making the thermometer’s quick, comfortable operation particularly valuable in pediatric care settings.
The device’s accuracy across the full pediatric temperature range ensures reliable fever detection and treatment monitoring. Age-appropriate correction factors and normal temperature ranges are programmed into connected AI systems, providing pediatric-specific clinical decision support.
Parental education programs can incorporate the NC 200’s home monitoring capabilities, enabling families to track their children’s temperatures accurately and communicate effectively with healthcare providers through integrated telehealth platforms.
Geriatric Care Applications
Geriatric patients often present unique challenges in temperature assessment, including altered thermoregulation, medication effects, and comorbid conditions that may affect temperature patterns. The NC 200’s precision and AI integration capabilities address these challenges through advanced analytics and pattern recognition.
The thermometer’s gentle operation and rapid measurement completion minimize stress for elderly patients who may have difficulty tolerating prolonged procedures. Integration with electronic health records enables tracking of temperature trends over time, facilitating early detection of health changes common in geriatric populations.
Nursing home and long-term care facility applications benefit from the device’s reliability and integration capabilities, enabling efficient monitoring of multiple residents while maintaining detailed health records for regulatory compliance and quality assurance.
Microlife Swiss Heritage and Engineering Excellence

Figure 6: Microlife Swiss medical technology heritage timeline showcasing precision engineering excellence and innovation leadership since 1981
Microlife Corporation’s legacy of Swiss engineering excellence spans over four decades, establishing the company as a global leader in medical device innovation and precision manufacturing. Founded in 1981 in Widnau, Switzerland, Microlife has consistently demonstrated its commitment to advancing healthcare technology through rigorous research, development, and quality assurance practices.
Company Foundation and Early Innovation (1981-1990)
Microlife Corporation was established with a vision to revolutionize medical device technology through Swiss precision engineering and innovative design. The company’s founders recognized the growing need for accurate, reliable medical instruments that could meet the demanding requirements of modern healthcare while remaining accessible to healthcare providers worldwide.
During its formative years, Microlife invested heavily in research and development, establishing state-of-the-art manufacturing facilities in Widnau, Switzerland. The company’s commitment to quality and precision quickly gained recognition within the medical device industry, leading to partnerships with healthcare institutions across Europe and beyond.
Early product development focused on temperature measurement technologies, with Microlife pioneering several innovations in digital thermometry that would become industry standards. The company’s emphasis on accuracy, reliability, and user-friendly design established its reputation for excellence that continues today.
International Expansion and Technology Leadership (1990-2000)
The 1990s marked a period of significant growth and international expansion for Microlife. The company established distribution networks across multiple continents, bringing Swiss-engineered medical devices to healthcare providers in North America, Asia, and emerging markets worldwide.
During this period, Microlife invested in advanced manufacturing technologies and quality assurance systems that would set new standards for medical device production. The company’s ISO 9001 certification and compliance with international medical device regulations demonstrated its commitment to quality and patient safety.
Technological innovations during this decade included advances in digital sensor technology, improved display systems, and enhanced ergonomic designs that improved healthcare provider satisfaction and patient comfort. Microlife’s products gained recognition for their accuracy, durability, and ease of use.
Digital Innovation and Connectivity (2000-2010)
The new millennium brought opportunities for Microlife to integrate digital technologies and connectivity features into its medical devices. The company recognized the growing importance of data management and electronic health records in modern healthcare, leading to investments in digital integration capabilities.
Advanced manufacturing processes implemented during this period enabled higher precision and consistency in device production. Microlife’s commitment to research and development resulted in numerous patents and technological innovations that advanced the field of medical thermometry.
Quality management systems evolved to meet increasingly stringent regulatory requirements, including FDA approval processes and European medical device directives. Microlife’s proactive approach to regulatory compliance ensured continued market access and customer confidence.
AI Integration and Modern Healthcare Solutions (2010-Present)
The current era of Microlife’s development focuses on artificial intelligence integration, cloud connectivity, and advanced healthcare analytics. The company’s partnership with leading AI platform providers enables healthcare organizations to leverage cutting-edge technology for improved patient care and operational efficiency.
Modern manufacturing facilities incorporate Industry 4.0 principles, including automated quality control, real-time monitoring, and predictive maintenance systems. These advances ensure consistent product quality while improving manufacturing efficiency and sustainability.
Microlife’s commitment to innovation continues through ongoing research and development programs that focus on emerging healthcare technologies, including telemedicine, remote patient monitoring, and personalized healthcare solutions.
Swiss Engineering Principles
Microlife’s adherence to traditional Swiss engineering principles ensures exceptional quality and reliability in every product. These principles include precision manufacturing, rigorous testing, continuous improvement, and unwavering attention to detail throughout the product development and production process.
The company’s manufacturing facilities in Widnau, Switzerland, represent the pinnacle of medical device production technology. Advanced precision machinery, climate-controlled environments, and highly trained technicians work together to produce devices that meet the most demanding quality standards.
Quality assurance processes incorporate multiple levels of testing and validation, from raw material inspection through final product certification. Each NC 200 Digital Thermometer undergoes individual calibration and testing to ensure accuracy and reliability before shipment to customers worldwide.
Research and Development Excellence
Microlife’s research and development programs focus on advancing medical device technology through collaboration with leading healthcare institutions, universities, and technology partners. The company’s investment in R&D represents a significant percentage of annual revenue, demonstrating its commitment to innovation and technological leadership.
Clinical validation studies conducted in partnership with medical institutions worldwide provide evidence of device performance and clinical utility. These studies contribute to peer-reviewed publications and regulatory submissions that support product approvals and clinical adoption.
Emerging technology partnerships, including collaborations with AI platform providers and healthcare analytics companies, position Microlife at the forefront of digital health innovation. These partnerships enable the development of integrated solutions that address evolving healthcare needs.
Global Manufacturing and Quality Standards
Microlife’s manufacturing operations maintain compliance with the most stringent international quality and regulatory standards. ISO 13485 certification for medical device quality management systems ensures consistent quality and regulatory compliance across all manufacturing locations.
Environmental management systems implemented throughout Microlife’s operations demonstrate the company’s commitment to sustainability and responsible manufacturing practices. These systems minimize environmental impact while maintaining the highest product quality standards.
Continuous improvement programs incorporate lean manufacturing principles, statistical process control, and employee training initiatives that enhance quality, efficiency, and customer satisfaction. These programs ensure that Microlife products continue to meet evolving healthcare needs and expectations.
Clinical Validation and Regulatory Compliance
The Microlife NC 200 Digital Thermometer has undergone extensive clinical validation to demonstrate its accuracy, reliability, and safety across diverse patient populations and clinical applications. These validation studies provide evidence of the device’s performance characteristics and support its regulatory approvals worldwide.
Accuracy and Precision Studies
Clinical accuracy studies have evaluated the NC 200’s performance against reference standard thermometry methods, including laboratory-grade mercury thermometers and calibrated electronic reference devices. These studies demonstrate the thermometer’s accuracy within ±0.1°C across the full clinical measurement range.
Precision studies have assessed the device’s repeatability and reproducibility under various clinical conditions. Results indicate excellent measurement consistency, with coefficient of variation values well within acceptable limits for clinical thermometry applications.
Comparative studies with other digital thermometers have consistently shown the NC 200’s superior accuracy and reliability. These studies provide healthcare providers with confidence in the device’s clinical performance and measurement validity.
Safety and Biocompatibility Assessment
Comprehensive safety evaluations have confirmed the NC 200’s biocompatibility and safety for patient contact applications. Materials testing according to ISO 10993 standards demonstrates the device’s suitability for oral, axillary, and rectal temperature measurement.
Electrical safety testing according to IEC 60601-1 standards ensures patient and operator protection from electrical hazards. The device’s battery-powered operation eliminates risks associated with line-powered medical equipment.
Risk analysis and hazard assessment procedures identify and mitigate potential safety concerns throughout the device’s lifecycle. These assessments ensure that the NC 200 meets the highest safety standards for medical device applications.
Regulatory Approvals and Certifications
The NC 200 has received regulatory approval from major health authorities worldwide, including the U.S. Food and Drug Administration (FDA), European Conformity (CE) marking, and Health Canada approval. These approvals demonstrate compliance with applicable medical device regulations and standards.
ISO 13485 quality management system certification ensures consistent quality throughout the device’s design, development, and manufacturing processes. This certification provides assurance of regulatory compliance and quality assurance practices.
Ongoing post-market surveillance programs monitor device performance and safety in real-world clinical applications. These programs ensure continued compliance with regulatory requirements and facilitate continuous improvement initiatives.
User Training and Clinical Implementation
Successful implementation of the Microlife NC 200 Digital Thermometer in clinical settings requires comprehensive user training and support programs. Microlife provides extensive resources to ensure healthcare providers can maximize the device’s capabilities and achieve optimal patient care outcomes.
Healthcare Provider Training Programs
Comprehensive training programs cover proper device operation, measurement techniques, maintenance procedures, and troubleshooting protocols. These programs are available in multiple formats, including in-person training, online modules, and instructional materials.
Clinical competency assessments ensure that healthcare providers demonstrate proficiency in device operation and temperature measurement techniques. These assessments contribute to quality assurance and patient safety initiatives within healthcare organizations.
Continuing education programs provide updates on device enhancements, new features, and best practices for temperature monitoring in clinical applications. These programs help healthcare providers stay current with evolving technology and clinical protocols.
Integration with Clinical Workflows
Workflow analysis and optimization services help healthcare organizations integrate the NC 200 into existing clinical processes efficiently. These services identify opportunities for improvement and ensure seamless device adoption without disrupting patient care activities.
Electronic health record integration support facilitates data connectivity and ensures accurate documentation of temperature measurements. This integration improves clinical efficiency and supports evidence-based patient care protocols.
Quality assurance programs provide ongoing monitoring of device performance and user satisfaction. These programs identify opportunities for improvement and ensure continued excellence in patient care delivery.
Maintenance and Service Support
Microlife’s comprehensive maintenance and service support programs ensure optimal device performance throughout the NC 200’s operational lifecycle. These programs minimize downtime, maximize reliability, and provide cost-effective device management solutions.
Preventive Maintenance Programs
Scheduled maintenance protocols help healthcare organizations maintain device accuracy and reliability through regular calibration checks, cleaning procedures, and performance verification. These protocols extend device lifespan and ensure consistent clinical performance.
Calibration services provide traceability to national temperature standards, ensuring measurement accuracy and regulatory compliance. Regular calibration intervals are established based on device usage patterns and clinical requirements.
Replacement part availability and technical support services ensure rapid resolution of device issues and minimal impact on clinical operations. Comprehensive inventory management helps healthcare organizations maintain adequate device availability.
Technical Support and Customer Service
Multi-channel technical support provides healthcare providers with access to expert assistance through phone, email, and online support platforms. Experienced technical specialists offer prompt resolution of device issues and user questions.
Comprehensive documentation and user resources, including operation manuals, quick reference guides, and troubleshooting procedures, support effective device utilization and problem resolution.
Customer feedback programs facilitate continuous improvement of products and services based on user experiences and suggestions. These programs ensure that Microlife continues to meet evolving healthcare needs and expectations.
Pricing and Purchase Information
Professional Healthcare Pricing
Microlife NC 200 Digital Thermometer
Single Unit Price: $24.99
Bulk Pricing (50+ units): $19.99 each
Institutional Pricing (500+ units): $17.99 each
Package Includes:
- Microlife NC 200 Digital Thermometer
- Protective storage case
- User manual and quick reference guide
- 1.5V LR41 battery (pre-installed)
- Calibration certificate
- 2-year manufacturer warranty
AI Integration Services:
Cloud Platform Integration: Starting at $199/month per facility
Custom AI Analytics Package: $499/month per facility
Enterprise Solution: Contact for pricing
Additional Services:
- Training and Implementation Support: $150/hour
- Annual Calibration Service: $15/device
- Extended Warranty (3rd year): $8.99/device
- Technical Support Contract: $99/year per facility
Free shipping on orders over $500 | Same-day shipping available | Volume discounts available for large orders
Conclusion
The Microlife NC 200 Digital Thermometer represents the pinnacle of Swiss engineering excellence in digital temperature measurement technology. Its integration with nine major AI cloud platforms positions it at the forefront of modern healthcare innovation, providing healthcare providers with unprecedented capabilities for patient monitoring, data analysis, and clinical decision support.
The device’s flexible tip design, advanced digital sensor technology, and rapid measurement capability make it suitable for diverse clinical applications while ensuring patient comfort and measurement accuracy. The comprehensive AI integration enables healthcare organizations to leverage cutting-edge technology for improved patient outcomes and operational efficiency.
Microlife’s four-decade legacy of Swiss precision engineering and commitment to quality assurance ensures that the NC 200 meets the most demanding healthcare requirements. The company’s ongoing investment in research and development, combined with strategic partnerships with leading AI platform providers, positions the NC 200 as a cornerstone of modern digital health infrastructure.
Healthcare providers seeking reliable, accurate, and technologically advanced temperature measurement solutions will find the Microlife NC 200 Digital Thermometer to be an invaluable addition to their clinical toolkit. Its combination of proven Swiss engineering, innovative AI integration, and comprehensive support services makes it the optimal choice for modern healthcare applications.
Contact Information:
Email: sales@medicaleq-shop.com
Phone: 1-800-MEDICAL (1-800-633-4225)
Website: www.medicaleq-shop.com
Live Chat: Available 24/7 for immediate assistance