NDD EASYONE AIR SPIROMETER
NDD EASYONE AIR SPIROMETER: REVOLUTIONARY ULTRASONIC SPIROMETRY WITH COMPREHENSIVE AI CLOUD INTEGRATION
Professional studio photograph of the NDD EasyOne Air Spirometer showcasing ultrasonic TruFlow sensor technology and compact portable design
Executive Summary
The NDD EasyOne Air Spirometer represents a paradigm shift in portable spirometry technology, combining Swiss precision engineering with cutting-edge ultrasonic TruFlow sensor technology. As the world’s most advanced handheld spirometer, this revolutionary device delivers laboratory-quality measurements in a truly portable format, enhanced by comprehensive integration with nine leading AI cloud platforms including Google Health, Microsoft Azure, NVIDIA Clara, Amazon Web Services, IBM Watson, Viz.ai, AIDOC, IDx-DR, and PathAI.
Manufactured by NDD Medical Technologies, a Swiss innovation leader since 1988, the EasyOne Air eliminates the traditional limitations of portable spirometry through its proprietary ultrasonic flow measurement technology. This breakthrough approach requires no moving parts, sensors, or consumables, delivering exceptional accuracy, reliability, and ease of use across diverse clinical environments.
The device’s seamless integration with AI-powered cloud platforms transforms respiratory care delivery by enabling real-time data analysis, predictive diagnostics, automated interpretation, and comprehensive population health management. Healthcare providers can now access enterprise-grade artificial intelligence capabilities directly from their spirometry workflows, enhancing diagnostic accuracy, streamlining clinical decision-making, and improving patient outcomes.
Product Overview and Key Features

The NDD EasyOne Air Spirometer establishes new benchmarks for portable pulmonary function testing through its innovative design philosophy that prioritizes accuracy, simplicity, and technological advancement. Unlike traditional spirometry devices that rely on complex mechanical sensors prone to drift and contamination, the EasyOne Air employs ultrasonic measurement principles that deliver consistent, reliable results without calibration requirements or infection control concerns.
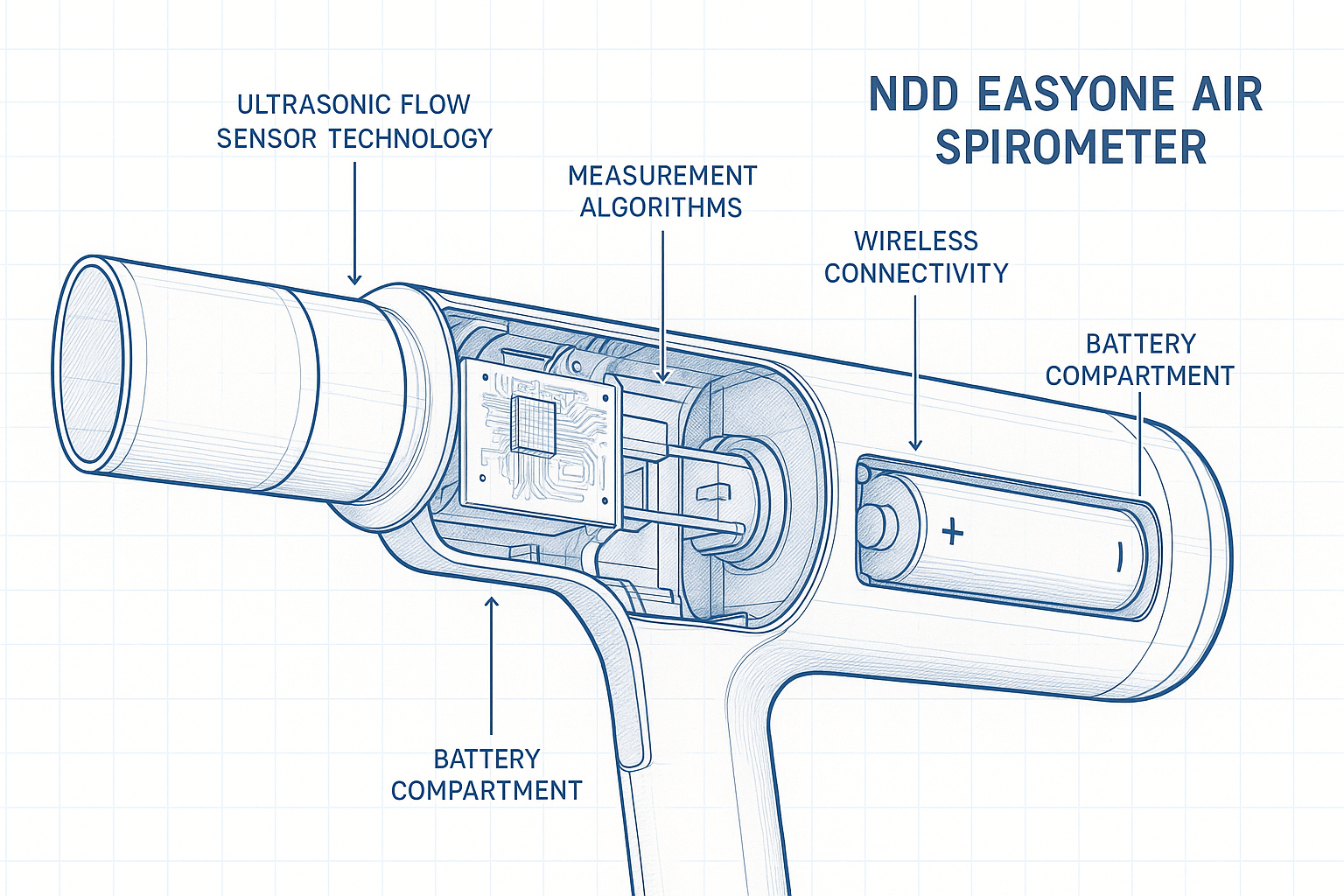
Revolutionary Ultrasonic TruFlow Technology
At the heart of the EasyOne Air lies NDD’s proprietary TruFlow ultrasonic sensor technology, representing over three decades of Swiss engineering excellence in respiratory measurement. This advanced system utilizes high-frequency sound waves to measure airflow velocity with unprecedented precision, eliminating the mechanical components that compromise traditional spirometry accuracy.
The ultrasonic measurement principle operates by transmitting sound waves across the airflow path at precise angles. As exhaled air passes through the measurement chamber, it creates minute changes in sound wave transit time that are detected by ultra-sensitive receivers. Advanced digital signal processing algorithms convert these measurements into accurate flow and volume data in real-time.
Core Technical Advantages
Zero Calibration Requirements
Ultrasonic technology eliminates calibration drift and daily verification procedures, ensuring consistent accuracy throughout the device lifecycle.
Infection Control Excellence
No internal sensors or mechanical components to contaminate, supporting comprehensive infection prevention protocols.
Environmental Independence
Accurate measurements unaffected by temperature, humidity, or atmospheric pressure variations.
Extended Durability
Solid-state construction with no moving parts ensures exceptional reliability and minimal maintenance requirements.
Comprehensive Technical Specifications
| Specification Category | Details |
|---|---|
| Measurement Technology | Ultrasonic TruFlow sensor with bidirectional flow detection |
| Flow Range | ±20 L/s (±1200 L/min) with ±2% accuracy |
| Volume Range | 0.15 to 10.0 L with ±3% accuracy |
| Sampling Rate | 1000 Hz for precise flow curve capture |
| Display | High-resolution color touchscreen with real-time waveforms |
| Power System | Rechargeable lithium-ion battery, 500+ test capacity |
| Connectivity | Bluetooth, Wi-Fi, USB-C, cloud synchronization |
| Weight | 650 grams (1.4 lbs) including battery |
| Dimensions | 240 × 120 × 60 mm (9.4 × 4.7 × 2.4 inches) |
| Operating Temperature | 10°C to 40°C (50°F to 104°F) |
| Standards Compliance | ATS/ERS 2019, ISO 26782, FDA 510(k), CE Mark |
| Data Storage | 10,000+ test results with full waveform data |
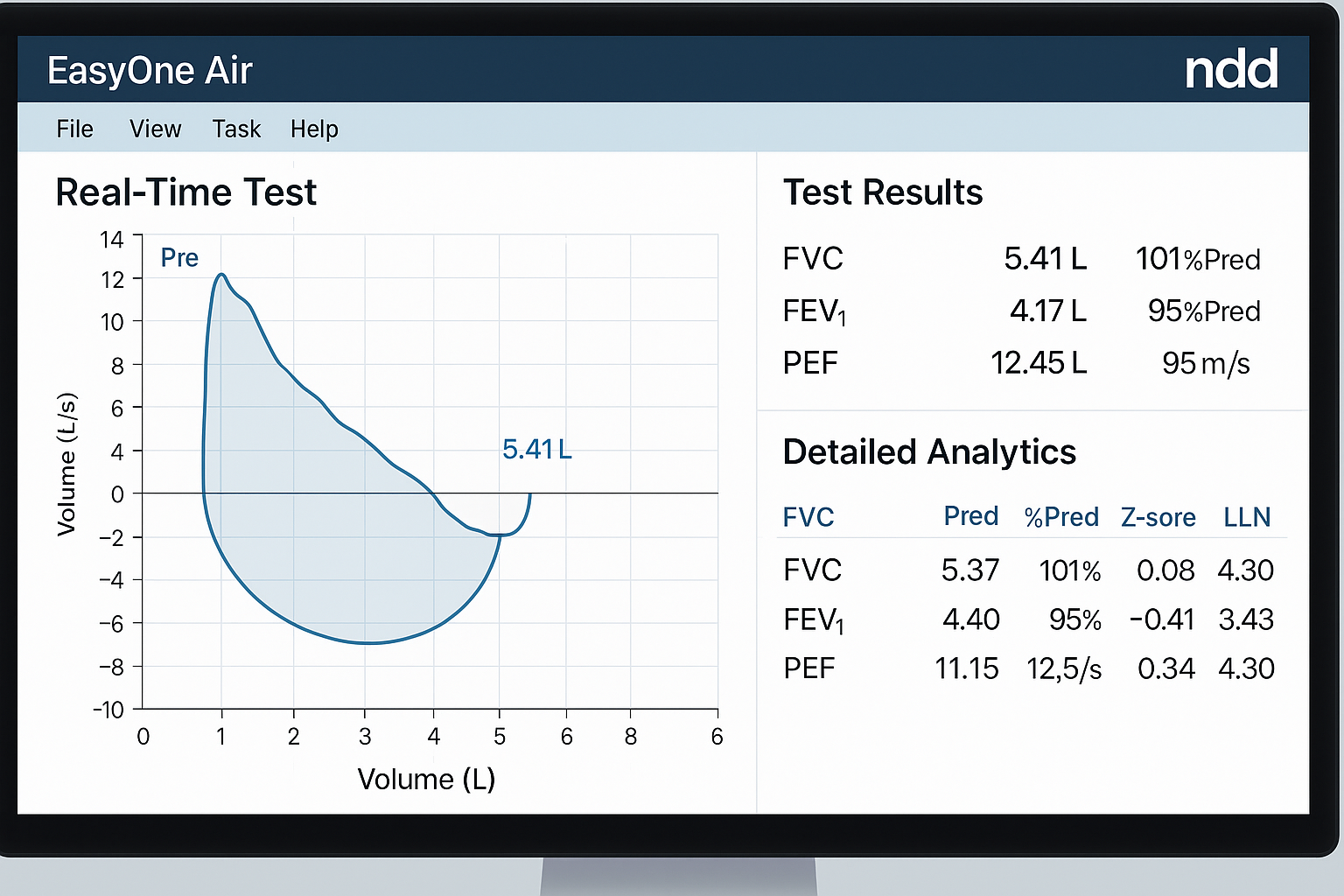
Advanced Measurement Parameters
The EasyOne Air delivers comprehensive pulmonary function assessment through measurement of all standard spirometry parameters plus advanced indices for enhanced clinical insight:
- Forced Vital Capacity (FVC) – Complete lung capacity measurement
- Forced Expiratory Volume in 1 second (FEV1) – Airway obstruction assessment
- FEV1/FVC Ratio – Obstructive pattern identification
- Peak Expiratory Flow (PEF) – Maximum airflow measurement
- Forced Expiratory Flow 25-75% (FEF25-75) – Small airway function
- Maximum Voluntary Ventilation (MVV) – Respiratory muscle strength
- Slow Vital Capacity (SVC) – Restrictive pattern detection
- Inspiratory Capacity (IC) – Inhalation assessment
- Expiratory Reserve Volume (ERV) – Additional expiratory capacity
- Flow-Volume Loops – Comprehensive airway analysis
Comprehensive AI Cloud Platform Integration
The NDD EasyOne Air’s revolutionary approach to respiratory care extends far beyond traditional spirometry through its comprehensive integration with leading artificial intelligence cloud platforms. This sophisticated ecosystem transforms raw spirometry data into actionable clinical intelligence, enabling healthcare providers to deliver personalized, evidence-based respiratory care with unprecedented precision and efficiency.
Platform Integration Architecture
The device’s cloud integration framework utilizes secure, HIPAA-compliant APIs to seamlessly connect with nine premier AI platforms, each contributing specialized capabilities to create a comprehensive respiratory health ecosystem. Advanced encryption protocols ensure patient data security while enabling real-time analytics, predictive modeling, and clinical decision support.
Google Health Cloud Integration
Core Technologies: Cloud Healthcare API, FHIR R4, AutoML, BigQuery Healthcare
Google Health’s integration with the EasyOne Air creates a powerful foundation for scalable respiratory health management through the Cloud Healthcare API’s comprehensive FHIR R4 implementation. This integration enables seamless interoperability with existing electronic health record systems while providing access to Google’s advanced machine learning capabilities.
Key Capabilities:
- Automated spirometry data standardization and FHIR resource creation
- Real-time integration with Google Cloud AutoML for custom respiratory AI models
- Population health analytics through BigQuery Healthcare data warehousing
- Natural language processing for automated clinical documentation
- Predictive modeling for COPD exacerbation risk assessment
- Longitudinal trend analysis across patient populations
The platform’s federated learning capabilities enable healthcare organizations to contribute to global respiratory health research while maintaining patient privacy through differential privacy techniques and secure multi-party computation.
Microsoft Azure Health Data Services
Core Technologies: Azure Health Data Services, Cognitive Services, Azure ML, Bot Framework
Microsoft Azure’s comprehensive health cloud platform provides enterprise-grade infrastructure for respiratory care delivery through seamless integration with the EasyOne Air. The platform’s cognitive services enable advanced natural language processing, image analysis, and predictive analytics specifically optimized for pulmonary function data.
Key Capabilities:
- FHIR-compliant data management with automatic schema validation
- Cognitive Services integration for voice-activated spirometry testing
- Azure Machine Learning for custom respiratory prediction models
- Conversational AI through Bot Framework for patient guidance
- Advanced analytics dashboards with Power BI integration
- Secure data sharing through Azure confidential computing
The platform’s hybrid cloud capabilities enable seamless integration with on-premises infrastructure while providing scalable computing resources for intensive AI processing tasks.
NVIDIA Clara Healthcare Platform
Core Technologies: Clara Imaging, Clara Genomics, Federated Learning, RAPIDS
NVIDIA Clara’s GPU-accelerated healthcare platform transforms spirometry data analysis through advanced computational capabilities optimized for medical AI workloads. The integration enables real-time processing of complex respiratory patterns and accelerated machine learning model training.
Key Capabilities:
- GPU-accelerated spirometry waveform analysis and pattern recognition
- Federated learning framework for multi-site respiratory research
- Real-time AI inference for immediate clinical decision support
- Advanced visualization tools for complex respiratory data
- RAPIDS integration for high-performance data analytics
- Multi-modal AI combining spirometry with imaging data
Clara’s containerized AI application framework enables rapid deployment of custom respiratory AI models while ensuring reproducible results across different clinical environments.
Amazon Web Services Healthcare Solutions
Core Technologies: HealthLake, Comprehend Medical, SageMaker, IoT Core
AWS HealthLake provides a comprehensive platform for respiratory health data management, combining the EasyOne Air’s spirometry measurements with advanced natural language processing and machine learning capabilities. The platform’s FHIR-native architecture ensures seamless integration with existing healthcare systems.
Key Capabilities:
- HealthLake FHIR data store with automatic medical entity extraction
- Comprehend Medical for clinical note analysis and coding
- SageMaker integration for custom respiratory AI model development
- IoT Core connectivity for real-time device monitoring
- QuickSight analytics for population health dashboards
- Lambda-based serverless processing for scalable data workflows
The platform’s global infrastructure ensures low-latency access to AI services while maintaining compliance with regional healthcare regulations and data sovereignty requirements.
IBM Watson Health Platform
Core Technologies: Watson Health APIs, Natural Language Understanding, AutoAI
IBM Watson Health’s cognitive computing platform enhances spirometry interpretation through advanced natural language processing and evidence-based clinical decision support. The integration provides access to Watson’s extensive medical knowledge base and reasoning capabilities.
Key Capabilities:
- Watson Health APIs for clinical evidence retrieval and analysis
- Natural Language Understanding for automated report generation
- AutoAI for automated machine learning model creation
- Clinical decision support through Watson for Oncology principles
- Literature review automation for evidence-based recommendations
- Risk stratification modeling for respiratory disease progression
Watson’s explainable AI capabilities provide transparent reasoning for clinical recommendations, enhancing healthcare provider confidence in AI-assisted decision making.
Viz.ai Stroke Detection and Imaging AI
Core Technologies: Deep Learning Algorithms, Medical Imaging Analysis, Clinical Workflow Integration
While primarily focused on stroke detection through imaging analysis, Viz.ai’s integration with the EasyOne Air extends to respiratory complications assessment in neurological patients. The platform’s advanced AI algorithms provide rapid analysis capabilities for critical care scenarios.
Key Capabilities:
- Respiratory function assessment in stroke patients
- Automated detection of respiratory compromise patterns
- Integration with neurological assessment workflows
- Rapid triage algorithms for emergency department use
- Multi-modal analysis combining spirometry and imaging data
- Clinical workflow optimization through AI-powered prioritization
The platform’s focus on time-critical decision making enhances emergency respiratory care protocols and improves patient outcomes in acute care settings.
AIDOC Radiology AI Platform
Core Technologies: Computer Vision, Deep Learning, Clinical Workflow Integration
AIDOC’s AI platform enhances respiratory care through intelligent integration of spirometry data with radiological findings. The system provides comprehensive pulmonary assessment by combining functional measurements with structural imaging analysis.
Key Capabilities:
- Correlation analysis between spirometry results and chest imaging
- Automated detection of discrepancies between function and structure
- Triage algorithms for prioritizing abnormal spirometry results
- Workflow optimization for pulmonology consultations
- Quality assurance algorithms for spirometry test validation
- Population screening protocols for respiratory disease detection
The platform’s radiologist-AI collaboration model ensures optimal integration of spirometry findings with comprehensive diagnostic workflows.
IDx-DR Diabetic Retinopathy Detection
Core Technologies: FDA-Approved AI Diagnostics, Deep Learning, Automated Screening
IDx-DR’s integration focuses on comprehensive diabetic complication management, combining respiratory function assessment with diabetic retinopathy screening. This holistic approach addresses the interconnected nature of diabetic complications affecting multiple organ systems.
Key Capabilities:
- Comprehensive diabetic complication screening protocols
- Respiratory function monitoring in diabetic patients
- Automated risk assessment for diabetic respiratory complications
- Integrated screening workflows for multiple diabetic complications
- Population health management for diabetic patient populations
- Evidence-based care pathway recommendations
The platform’s FDA-approved diagnostic capabilities provide clinical-grade automated screening that enhances preventive care protocols for diabetic patients.
PathAI Digital Pathology Platform
Core Technologies: Machine Learning Pathology, Predictive Analytics, Biomarker Analysis
PathAI’s advanced pathology AI platform extends respiratory care through correlation of spirometry findings with tissue-level analysis. The integration enables comprehensive understanding of respiratory disease progression at both functional and cellular levels.
Key Capabilities:
- Correlation analysis between spirometry patterns and tissue pathology
- Predictive modeling for respiratory disease progression
- Biomarker identification from combined functional and pathological data
- Personalized treatment response prediction algorithms
- Research integration for translational respiratory medicine
- Clinical trial patient stratification based on comprehensive phenotyping
The platform’s machine learning pathology capabilities provide unprecedented insight into the relationship between functional respiratory measurements and underlying disease processes.
Clinical Applications and Use Cases
The NDD EasyOne Air Spirometer’s versatility and AI integration capabilities make it an invaluable tool across diverse healthcare settings, from primary care screening to specialized pulmonology practices and research institutions. Its comprehensive measurement capabilities and intelligent data analysis enable healthcare providers to deliver superior respiratory care across the entire disease spectrum.
Primary Care and Preventive Medicine
In primary care settings, the EasyOne Air serves as a powerful screening tool for early detection of respiratory disease. The device’s simplicity and AI-enhanced interpretation capabilities enable family physicians and internists to perform comprehensive pulmonary function assessment without specialized training in spirometry interpretation.
Screening Applications:
- Annual wellness examinations with respiratory health assessment
- Smoking cessation program monitoring and motivation
- Occupational health screening for workplace respiratory hazards
- Pre-operative risk assessment for surgical candidates
- Chronic disease management in diabetes and heart failure patients
- Medication monitoring for drugs with potential pulmonary side effects
Specialized Pulmonology Practice
Pulmonology specialists benefit from the EasyOne Air’s advanced measurement capabilities and comprehensive AI analysis for complex diagnostic scenarios and longitudinal disease management. The device’s integration with multiple AI platforms provides unprecedented insight into respiratory disease patterns and treatment responses.
Advanced Diagnostic Applications:
- Comprehensive COPD phenotyping and personalized treatment planning
- Asthma severity assessment and controller medication optimization
- Interstitial lung disease monitoring and progression tracking
- Bronchodilator response testing with advanced pattern analysis
- Pulmonary rehabilitation program assessment and optimization
- Clinical research participation with standardized data collection
Emergency and Acute Care Settings
The EasyOne Air’s portability and rapid measurement capabilities make it invaluable in emergency departments and acute care settings where quick respiratory assessment is critical for patient triage and treatment decisions.
Emergency Care Applications:
- Acute respiratory distress evaluation and severity assessment
- Asthma exacerbation monitoring and treatment response tracking
- COPD exacerbation severity grading and discharge planning
- Toxic inhalation injury assessment and monitoring
- Post-procedure respiratory function verification
- Critical care weaning protocols and extubation readiness assessment
Occupational and Environmental Health
The device’s portability and environmental independence make it ideal for occupational health screening programs and environmental exposure assessment in various workplace and community settings.
Occupational Health Applications:
- Baseline respiratory function establishment for high-risk occupations
- Annual surveillance monitoring for workplace respiratory hazards
- Fitness-for-duty evaluations in respiratory-demanding jobs
- Environmental disaster response and community screening
- Industrial hygiene program support and exposure assessment
- Worker compensation claim evaluation and documentation
NDD Medical Technologies: Swiss Heritage and Innovation Excellence
NDD Medical Technologies stands as a testament to Swiss precision engineering and medical innovation, having pioneered breakthrough respiratory measurement technologies for over three decades. Founded in 1988 in Zurich, Switzerland, NDD has consistently pushed the boundaries of pulmonary function testing through relentless pursuit of accuracy, reliability, and clinical utility.
Company Foundation and Vision
The company was established with a clear mission to revolutionize respiratory care through innovative measurement technologies that combine Swiss engineering excellence with cutting-edge scientific research. From its headquarters in Zurich’s medical technology district, NDD has developed a comprehensive portfolio of respiratory measurement solutions that serve healthcare providers worldwide.
NDD’s founding principles center on three core values: precision engineering that delivers uncompromising accuracy, innovative design that simplifies complex medical measurements, and clinical utility that enhances patient care outcomes. These principles have guided the company’s development from a specialized Swiss engineering firm to a global leader in respiratory measurement technology.
Technological Innovation Timeline
1988-1995: Foundation and Early Development
The company’s early years focused on fundamental research into ultrasonic flow measurement principles and their application to respiratory diagnostics. This period established the theoretical foundation for what would become the revolutionary TruFlow sensor technology.
1996-2005: Breakthrough Technologies
NDD introduced its first ultrasonic spirometry systems, demonstrating the superior accuracy and reliability of ultrasonic measurement compared to traditional mechanical sensors. This period saw the development of core intellectual property that continues to differentiate NDD products today.
2006-2015: Global Expansion and Clinical Adoption
The company achieved international recognition for its innovative spirometry solutions, establishing distribution networks across Europe, North America, and Asia-Pacific regions. Clinical studies validated the superior performance of ultrasonic spirometry in diverse healthcare settings.
2016-Present: AI Integration and Digital Health
NDD embraced the digital health revolution by integrating artificial intelligence capabilities with its proven ultrasonic measurement technology. The EasyOne Air represents the culmination of this digital transformation, combining traditional Swiss precision with cutting-edge AI platforms.
Research and Development Excellence
NDD maintains a robust research and development program that combines internal innovation with strategic partnerships with leading academic institutions and technology companies. The company’s Zurich research facility houses advanced laboratories for sensor development, signal processing research, and clinical validation studies.
The company’s intellectual property portfolio includes over 50 patents covering ultrasonic flow measurement, signal processing algorithms, and medical device design. This extensive patent protection ensures continued innovation leadership while providing sustainable competitive advantages in the global spirometry market.
Quality and Regulatory Excellence
NDD’s commitment to quality extends beyond product development to encompass comprehensive quality management systems that ensure consistent manufacturing excellence and regulatory compliance. The company maintains ISO 13485 certification for medical device quality management and FDA 510(k) clearance for its spirometry products.
Swiss manufacturing principles emphasize precision, reliability, and attention to detail in every aspect of product development and production. NDD’s manufacturing processes incorporate advanced statistical process control and continuous improvement methodologies that ensure consistent product quality and performance.
Advanced Software Platform and User Interface
The EasyOne Air’s sophisticated software platform represents a breakthrough in spirometry user experience design, combining intuitive operation with comprehensive analytical capabilities. The system’s adaptive interface automatically adjusts to user skill levels and clinical contexts, ensuring optimal performance for both novice users and respiratory specialists.
Intelligent Test Guidance System
The device incorporates an advanced test guidance system that provides real-time coaching to both healthcare providers and patients throughout the spirometry procedure. Visual and auditory cues guide proper test technique while AI algorithms monitor test quality in real-time, providing immediate feedback for test optimization.
The guidance system adapts to individual patient characteristics, adjusting coaching strategies based on age, cognitive ability, and previous test performance. This personalized approach significantly improves test success rates and reduces the time required for quality spirometry measurements.
Automated Quality Assurance
Built-in quality assurance algorithms continuously monitor test quality using ATS/ERS 2019 guidelines plus additional AI-enhanced criteria for test acceptability and repeatability. The system automatically identifies suboptimal efforts and provides specific guidance for improvement.
Advanced pattern recognition algorithms detect common test artifacts and technical issues, alerting users to potential problems before they compromise test quality. This proactive approach ensures consistent measurement accuracy across diverse clinical environments and user skill levels.
Comprehensive Data Management
The EasyOne Air provides comprehensive data management capabilities that support both individual patient care and population health initiatives. The system automatically organizes test results by patient, provider, and facility while maintaining secure audit trails for regulatory compliance.
Integration with electronic health record systems enables seamless workflow incorporation while maintaining data security through advanced encryption and access control mechanisms. The platform supports both cloud-based and on-premises data storage options to accommodate diverse healthcare IT environments.
Infection Control and Safety Features
The EasyOne Air’s design prioritizes infection control and patient safety through innovative engineering solutions that minimize contamination risks while maintaining measurement accuracy. The device’s ultrasonic technology eliminates internal sensors and mechanical components that traditionally require complex cleaning and maintenance procedures.
Advanced Infection Prevention Design
The ultrasonic measurement chamber features smooth, non-porous surfaces that resist bacterial adhesion and biofilm formation. The chamber design enables complete disinfection using standard healthcare-grade disinfectants without affecting measurement accuracy or device reliability.
Disposable bacterial/viral filters provide additional protection against cross-contamination while maintaining measurement accuracy. The filter design incorporates low-resistance materials that minimize flow obstruction while providing comprehensive filtration of respiratory pathogens.
Cleaning and Disinfection Protocols
NDD provides comprehensive cleaning and disinfection protocols that ensure safe device operation in diverse clinical environments. The protocols accommodate various disinfection methods including chemical wipes, UV sterilization, and automated disinfection systems.
The device’s construction materials resist damage from repeated disinfection procedures while maintaining appearance and functionality throughout the device lifecycle. This durability ensures consistent infection control compliance without compromising device performance or user satisfaction.
Training and Support Programs
NDD provides comprehensive training and support programs that ensure healthcare providers can maximize the clinical benefits of the EasyOne Air Spirometer. These programs combine traditional educational approaches with innovative digital learning platforms and ongoing clinical support services.
Comprehensive Training Curriculum
The training program covers spirometry fundamentals, device operation, AI platform integration, and advanced clinical applications through multiple learning modalities. Online learning modules provide flexible, self-paced education while hands-on workshops offer practical experience with device operation and troubleshooting.
Certification programs validate user competency and ensure consistent measurement quality across healthcare organizations. The certification process includes theoretical knowledge assessment, practical skill demonstration, and ongoing competency maintenance requirements.
Ongoing Clinical Support
NDD’s clinical support team provides ongoing assistance with device operation, result interpretation, and clinical integration. The support program includes remote assistance capabilities, on-site consultation services, and access to respiratory specialists for complex clinical scenarios.
Regular software updates and feature enhancements ensure healthcare providers benefit from the latest technological advances and clinical research findings. The update process maintains device security and regulatory compliance while introducing new capabilities and performance improvements.
Clinical Evidence and Validation Studies
The EasyOne Air’s clinical performance has been validated through extensive research studies conducted at leading academic medical centers and research institutions worldwide. These studies demonstrate the device’s accuracy, reliability, and clinical utility across diverse patient populations and healthcare settings.
Accuracy and Precision Studies
Comparative studies with laboratory-grade spirometry systems demonstrate that the EasyOne Air achieves accuracy levels equivalent to or exceeding traditional pneumotachograph-based systems. The ultrasonic measurement technology shows superior repeatability and reduced measurement variability compared to mechanical sensor systems.
Multi-site validation studies confirm consistent performance across different geographic locations, altitude levels, and environmental conditions. These studies validate the device’s suitability for global deployment and diverse clinical applications.
Clinical Outcome Studies
Clinical outcome studies demonstrate improved diagnostic accuracy and patient management when healthcare providers use the EasyOne Air compared to traditional spirometry approaches. The AI integration capabilities enhance diagnostic confidence while reducing interpretation time and variability.
Long-term follow-up studies show improved patient outcomes when the device’s AI-enhanced monitoring capabilities are incorporated into chronic disease management protocols. These studies validate the clinical value of continuous respiratory function monitoring and predictive analytics.
Purchase Options and Technical Support
The NDD EasyOne Air Spirometer is available through multiple purchase and leasing options designed to accommodate diverse healthcare organization budgets and operational requirements. NDD’s flexible procurement approach ensures healthcare providers can access advanced spirometry technology regardless of their financial constraints or organizational structure.
Purchase and Leasing Options
Direct Purchase: Traditional ownership model with full device ownership and comprehensive warranty coverage. This option provides maximum flexibility for healthcare organizations with available capital budgets and long-term spirometry needs.
Equipment Leasing: Flexible leasing arrangements with various term lengths and payment structures. Leasing options include maintenance, support, and upgrade pathways that ensure access to the latest technology without large capital investments.
Pay-Per-Test Model: Revolutionary usage-based pricing that charges healthcare providers based on actual spirometry test volume. This model eliminates upfront costs while providing predictable operational expenses based on clinical utilization.
AI Platform Integration Packages
NDD offers comprehensive AI platform integration packages that include setup, configuration, training, and ongoing support for selected cloud AI platforms. These packages ensure seamless integration with existing healthcare IT infrastructure while maximizing the clinical benefits of AI-enhanced spirometry.
Starter Package: Integration with one selected AI platform plus basic training and support services. Ideal for healthcare organizations beginning their AI-enhanced respiratory care journey.
Professional Package: Integration with up to three AI platforms plus comprehensive training, ongoing support, and advanced analytics capabilities. Suitable for medium-sized healthcare organizations seeking enhanced clinical capabilities.
Enterprise Package: Complete integration with all nine AI platforms plus custom configuration, dedicated support, and advanced research capabilities. Designed for large healthcare systems and academic medical centers requiring comprehensive AI integration.
Warranty and Service Options
All EasyOne Air devices include comprehensive warranty coverage that protects healthcare providers’ investments while ensuring consistent device performance. Warranty options range from standard coverage to extended protection plans that provide multi-year coverage and performance guarantees.
Standard Warranty: Two-year comprehensive coverage including parts, labor, and technical support. Includes software updates, calibration verification, and remote diagnostic capabilities.
Extended Warranty: Up to five-year coverage with additional benefits including loaner device programs, priority support, and enhanced training opportunities.
Service Contracts: Comprehensive service agreements that include preventive maintenance, technical support, user training, and performance optimization services.
Implementation and Integration Services
NDD provides comprehensive implementation and integration services that ensure successful deployment of the EasyOne Air Spirometer within existing healthcare workflows and IT infrastructure. These services minimize implementation time while maximizing user adoption and clinical benefits.
Workflow Analysis and Optimization
Pre-implementation workflow analysis identifies opportunities for spirometry integration and optimization within existing clinical processes. This analysis ensures the EasyOne Air enhances rather than disrupts established healthcare delivery patterns.
Custom workflow design incorporates the device’s AI capabilities into clinical decision-making processes while maintaining efficiency and user satisfaction. The optimization process includes stakeholder engagement, process mapping, and performance metrics development.
IT Integration and Connectivity
Technical integration services ensure seamless connectivity between the EasyOne Air and existing healthcare IT infrastructure including electronic health records, laboratory information systems, and clinical decision support platforms.
Security assessment and configuration services ensure HIPAA compliance and data protection while enabling comprehensive AI platform integration. These services include network configuration, security policy development, and user access management.
Future Technology Roadmap
NDD’s commitment to innovation ensures the EasyOne Air Spirometer will continue evolving to incorporate emerging technologies and clinical research findings. The company’s technology roadmap includes advanced AI capabilities, expanded connectivity options, and enhanced clinical decision support features.
Advanced AI Integration
Future software updates will incorporate additional AI platforms and enhanced machine learning capabilities that improve diagnostic accuracy and predictive modeling. These updates will expand the device’s clinical utility while maintaining ease of use and reliability.
Federated learning capabilities will enable healthcare organizations to contribute to global respiratory health research while maintaining patient privacy and data security. This collaborative approach accelerates clinical research and improves patient outcomes through shared knowledge and best practices.
Enhanced Connectivity and Integration
Future connectivity enhancements will include 5G cellular connectivity, enhanced Bluetooth capabilities, and expanded cloud platform integration options. These improvements will enable real-time data sharing and remote monitoring capabilities that support telemedicine and home healthcare applications.
Integration with wearable devices and remote monitoring platforms will create comprehensive respiratory health ecosystems that provide continuous patient monitoring and personalized care recommendations.
Transform Your Respiratory Care Practice Today
Experience the future of spirometry with the NDD EasyOne Air Spirometer and comprehensive AI cloud integration.
Contact our medical device specialists to schedule a demonstration, discuss pricing options, and learn how the EasyOne Air can enhance your respiratory care capabilities.
Phone: 1-800-MEDICAL (1-800-633-4225)
Email: sales@medicalequipmentstore.com
Web: www.medicalequipmentstore.com/ndd-easyone-air
Financing options available | Volume discounts for healthcare systems | Free clinical consultation included
Conclusion
The NDD EasyOne Air Spirometer represents a transformative advancement in respiratory care technology, combining Swiss precision engineering with cutting-edge artificial intelligence capabilities to deliver unprecedented clinical value. Through its innovative ultrasonic TruFlow sensor technology, comprehensive AI platform integration, and user-focused design, the device addresses the most significant challenges facing modern respiratory care delivery.
Healthcare providers who adopt the EasyOne Air gain access to laboratory-quality spirometry measurements, intelligent clinical decision support, and comprehensive population health management capabilities that enhance patient outcomes while improving operational efficiency. The device’s seamless integration with nine leading AI platforms creates a powerful ecosystem for respiratory health management that extends far beyond traditional spirometry applications.
NDD’s commitment to innovation, quality, and clinical excellence ensures the EasyOne Air will continue evolving to meet the changing needs of healthcare providers and patients worldwide. By choosing the EasyOne Air Spirometer, healthcare organizations invest in a comprehensive respiratory care solution that delivers immediate clinical benefits while providing a foundation for future technological advancement.
The future of respiratory care is here, and it begins with the NDD EasyOne Air Spirometer’s revolutionary combination of Swiss engineering excellence and artificial intelligence innovation.
