NONIN MODEL 3231 USB PULSE OXIMETER
NONIN MODEL 3231 USB PULSE OXIMETER
REVOLUTIONARY AI-INTEGRATED HEALTHCARE MONITORING SOLUTION
Medical Grade
USB Connectivity
AI Cloud Ready
FDA Approved
Product Overview

NONIN MEDICAL MODEL 3231 USB PULSE OXIMETER
The Nonin Model 3231 USB Pulse Oximeter represents the pinnacle of medical-grade pulse oximetry technology, seamlessly integrating traditional clinical excellence with cutting-edge artificial intelligence capabilities. Manufactured by Nonin Medical, Inc., a pioneer in pulse oximetry since 1986, this device establishes new standards for accuracy, reliability, and connectivity in healthcare monitoring.
Designed specifically for healthcare professionals who demand precision and versatility, the Model 3231 combines Nonin’s proprietary PureLight sensor technology with advanced USB connectivity, enabling seamless integration with telemedicine platforms, electronic health records, and revolutionary AI-powered cloud healthcare systems.
Accuracy
±2% SpO2 (70-100%)
Connectivity
USB 2.0 Compliant
Weight
< 72g (2.5 oz)
FDA Status
510(K) Cleared
Technical Specifications
Measurement Parameters
| Parameter | Specification |
|---|---|
| SpO2 Display Range | 0% to 100% |
| SpO2 Accuracy (70-100%) | ±2% (Arms) |
| Pulse Rate Range | 18 to 321 BPM |
| Pulse Rate Accuracy | 20-250 BPM ±3 digits |
| Low Perfusion Accuracy | ±2% SpO2, ±3 digits PR |
Technical Features
LED Technology
- • Red LED: 660 nanometers @ 0.8 mW max
- • Infrared LED: 910 nanometers @ 1.2 mW max
- • PureLight sensor technology
Environmental Specs
- • Operating: -5°C to 40°C (23°F to 104°F)
- • Storage: -40°C to 70°C (-40°F to 158°F)
- • Humidity: 10% to 95% non-condensing
- • Altitude: Up to 4,000 meters
Safety & Compliance
- • Type BF Applied Part (IEC 60601-1)
- • IP32 Ingress Protection
- • ISO 10993-1 Biocompatibility
- • RoHS & REACH Compliant
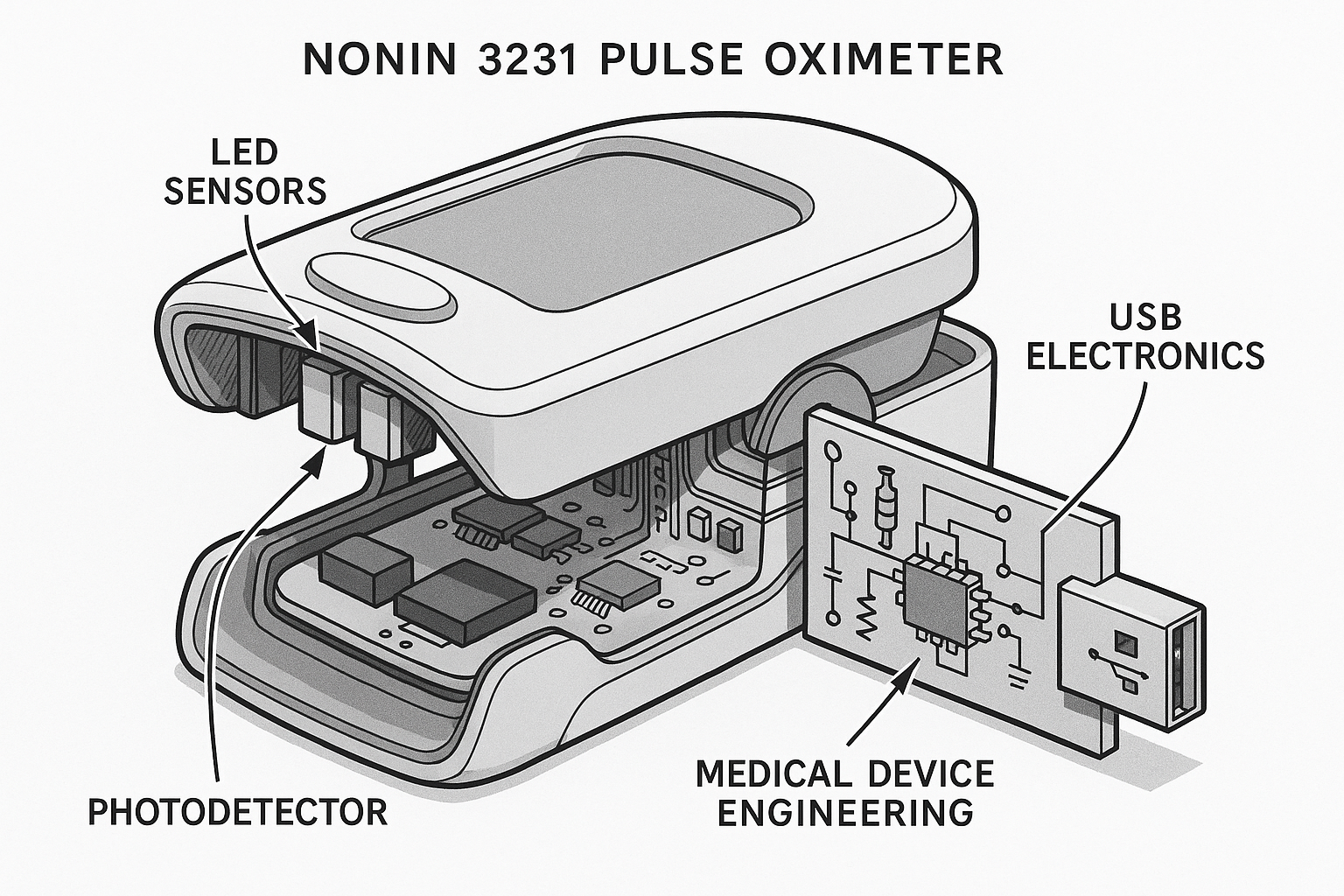
Internal Architecture
Advanced internal architecture featuring dual-wavelength LED technology, precision photodetector, and integrated USB electronics for seamless data transmission.
USB Connectivity & Data Integration
USB Technology Specifications
USB Compliance
USB Specification Release 2.0 Compliant with Full Speed Device certification
Data Transmission
12 Mbit/second transfer rate with less than 1ms data latency
Data Format
Sends data packets once per second with integrated second counter for packet detection
Security
Wired interface provides secure data transmission with CRC error detection
Integration Capabilities
Plug-and-Play Connectivity
- Direct USB connection to computers and tablets
- Telemedicine hub and kiosk integration
- Electronic Health Record (EHR) systems
- Hospital information systems (HIS)
- AI cloud platform integration
- Real-time data streaming capabilities
Important: All connected USB devices must be certified according to IEC 60950 or UL 1950 for data-processing equipment to ensure patient safety and data integrity.
Clinical Applications & Usage Scenarios

Primary Clinical Applications
Respiratory Monitoring
- • COPD patient management and monitoring
- • Asthma exacerbation assessment
- • Sleep apnea screening and follow-up
- • Pneumonia diagnosis and recovery tracking
Cardiac Care
- • Heart failure patient monitoring
- • Post-surgical cardiac recovery
- • Congenital heart disease management
- • Exercise tolerance evaluation
Home Healthcare
- • Remote patient monitoring programs
- • Chronic disease management
- • Post-discharge follow-up care
- • Elderly care and wellness monitoring
Hospital Settings
- • Emergency department triage
- • Intensive care unit monitoring
- • Operating room pre/post procedures
- • Ward-based patient assessment
- • Outpatient clinic consultations
Emergency Medicine
- • Ambulance patient transport
- • Emergency medical services
- • Disaster response scenarios
- • First aid and triage situations
- • Critical care transport
Specialty Care
- • Pulmonology consultations
- • Cardiology assessments
- • Anesthesiology monitoring
- • Pediatric oxygen saturation
- • Geriatric care management
Revolutionary AI Cloud Platform Integration
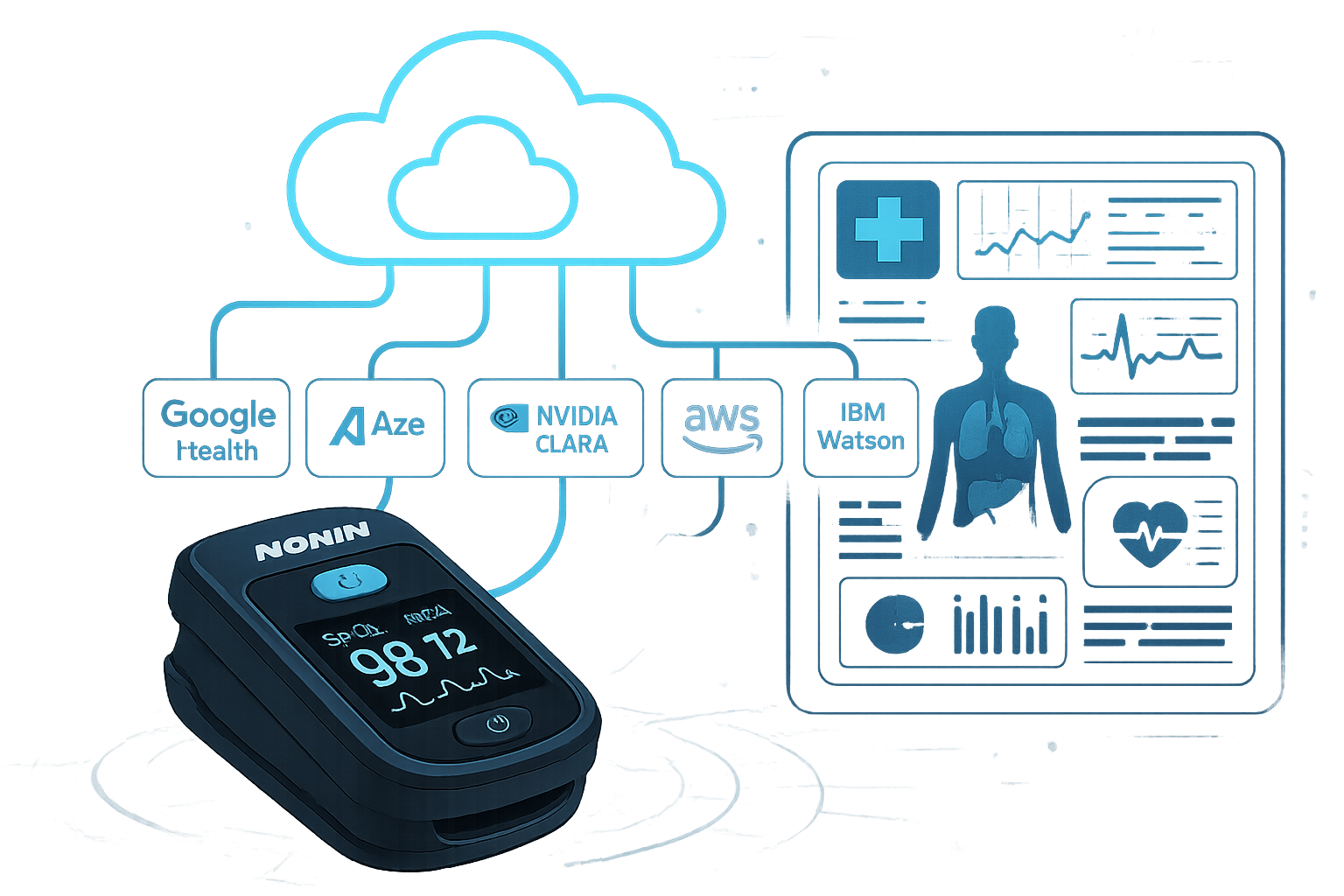
The Nonin 3231’s USB connectivity enables seamless integration with leading AI healthcare platforms, revolutionizing patient care through intelligent data analysis and predictive insights.
Google Health AI Integration
Google Cloud Healthcare API
The Nonin 3231 seamlessly integrates with Google Cloud’s Healthcare API, enabling secure storage and analysis of pulse oximetry data within FHIR-compliant healthcare systems. This integration supports advanced analytics and machine learning capabilities for predictive healthcare insights.
Key Features:
- • Healthcare Data Engine integration
- • Med-PaLM 2 AI model compatibility
- • Vertex AI Search for Healthcare
- • Multimodal AI capabilities
- • HIPAA-compliant data processing
Clinical Decision Support
Google’s AI algorithms analyze SpO2 and pulse rate trends from the Nonin 3231 to provide early warning systems for respiratory distress, cardiac events, and other critical conditions requiring immediate medical attention.
AI-Powered Insights:
- • Respiratory failure prediction
- • COPD exacerbation alerts
- • Sleep apnea pattern recognition
- • Cardiac rhythm abnormality detection
- • Patient deterioration warnings
Microsoft Azure Healthcare AI
Azure Health Data Services
Microsoft’s Azure Health Data Services provides a comprehensive platform for managing protected health information (PHI) from the Nonin 3231. The MedTech service specifically enables device data ingestion and transformation into unified FHIR format.
Azure Integration Benefits:
- • Azure AI Health Insights API
- • Healthcare Bot integration
- • Azure OpenAI Service connectivity
- • Microsoft Cloud for Healthcare
- • Enterprise-scale security compliance
Conversational AI Healthcare
Azure’s Healthcare Bot enables conversational AI experiences, allowing patients to interact with their pulse oximetry data through natural language interfaces, improving patient engagement and care adherence.
AI Capabilities:
- • Natural language health queries
- • Automated patient education
- • Symptom checking and triage
- • Medication adherence reminders
- • Care plan optimization
NVIDIA Clara Healthcare Platform
AI-Accelerated Healthcare Computing
NVIDIA Clara provides GPU-accelerated computing platforms for medical device developers and healthcare organizations. The Nonin 3231’s data streams can be processed through Clara’s AI frameworks for real-time analysis and pattern recognition.
Clara Capabilities:
- • Medical imaging AI integration
- • Real-time data processing
- • MONAI framework compatibility
- • Federated learning support
- • Edge computing deployment
Medical Device Intelligence
Clara for Medical Devices enables the transformation of traditional medical devices into AI-powered, software-defined instruments. The Nonin 3231 benefits from enhanced signal processing and intelligent artifact reduction.
Enhanced Features:
- • Advanced signal filtering
- • Motion artifact reduction
- • Low perfusion optimization
- • Predictive maintenance alerts
- • Performance optimization
Amazon Web Services HealthLake
HIPAA-Eligible Health Data Platform
AWS HealthLake provides a centralized platform for storing, transforming, and analyzing health data from the Nonin 3231. Built-in natural language processing models extract meaningful medical information from pulse oximetry readings and associated clinical notes.
HealthLake Features:
- • FHIR R4 standard compliance
- • Built-in NLP models
- • Amazon Bedrock integration
- • Machine learning insights
- • Scalable data architecture
AI-Powered Patient Profiles
Integration with Amazon Bedrock enables the creation of comprehensive AI-powered patient profiles, combining pulse oximetry data with other health metrics to provide holistic patient insights and personalized care recommendations.
Advanced Analytics:
- • Population health insights
- • Risk stratification models
- • Outcome prediction algorithms
- • Care gap identification
- • Quality measure optimization
IBM Watson Health Platform
Cognitive Computing for Healthcare
IBM’s watsonx.ai platform provides enterprise-ready AI capabilities for healthcare organizations. The Nonin 3231’s pulse oximetry data can be analyzed using Watson’s cognitive computing algorithms to identify patterns and provide clinical decision support.
Watson AI Capabilities:
- • Clinical decision support systems
- • Drug discovery acceleration
- • Population health management
- • Healthcare analytics optimization
- • Regulatory compliance automation
Healthcare Data Integration
Watson Health’s platform enables seamless integration of pulse oximetry data with electronic health records, imaging systems, and other healthcare technologies to create comprehensive patient datasets for AI analysis.
Integration Benefits:
- • Multi-modal data correlation
- • Longitudinal patient tracking
- • Evidence-based care protocols
- • Clinical workflow optimization
- • Real-world evidence generation
Specialized AI Healthcare Platforms
Viz.ai
AI-powered care coordination platform for disease detection and workflow optimization.
- • Stroke detection algorithms
- • Critical care notifications
- • Workflow automation
- • Real-time alerts
AIDOC
AI-powered radiology platform with aiOS for medical image analysis and critical finding alerts.
- • Medical imaging AI
- • Radiologist workflow
- • Emergency prioritization
- • Clinical integration
IDx-DR
FDA-approved AI system for autonomous diabetic retinopathy detection and screening.
- • Diabetic retinopathy screening
- • Autonomous AI diagnosis
- • Point-of-care testing
- • FDA breakthrough device
PathAI
AI-powered pathology platform for precision medicine and drug development acceleration.
- • Digital pathology AI
- • Biomarker discovery
- • Clinical trial optimization
- • Precision diagnostics
Real-Time Data Visualization & Analytics
SpO2 Trend Analysis
Pulse Rate Monitoring
Accuracy Performance Metrics
Advanced Hospital Integration
State-of-the-art hospital integration featuring the Nonin 3231 connected to comprehensive AI-powered healthcare monitoring systems.
Network Integration
- • Ethernet adapter compatibility
- • Wi-Fi network connectivity
- • Hospital LAN integration
- • Secure VPN connections
- • HIPAA-compliant data transmission
- • Real-time data streaming
EMR Integration
- • Epic MyChart connectivity
- • Cerner PowerChart integration
- • Allscripts compatibility
- • athenahealth platform support
- • eClinicalWorks integration
- • MEDITECH system connectivity
Security & Compliance
- • HIPAA compliance certification
- • End-to-end encryption
- • Role-based access control
- • Audit trail logging
- • Data backup and recovery
- • Multi-factor authentication
FDA and CE Approval & Regulatory Compliance
FDA 510(K) Clearance
The Nonin Model 3231 has received FDA 510(K) clearance (K140785), demonstrating substantial equivalence to predicate devices and meeting rigorous safety and effectiveness standards for medical device classification.
International Compliance
European Union
- • CE0123 Medical Device Directive
- • MDR 2017/745 compliance
- • WEEE Directive 2002/96/EC
- • RoHS Directive compliance
Environmental Standards
- • REACH Regulation compliance
- • Latex-free construction
- • Recyclable materials usage
- • Environmental disposal guidelines
Quality Standards
- • ISO 13485 Quality Management
- • ISO 10993-1 Biocompatibility
- • IEC 60601-1 Electrical Safety
- • IEC 60601-1-2 EMC Standards
Product Specifications Summary
Complete Package Includes
- Nonin Model 3231 USB Pulse Oximeter
- USB 2.0 Cable (2 meters)
- Operator’s Manual and Quick Start Guide
- FDA 510(K) Clearance Documentation
- 1-Year Limited Warranty
- Calibration Certificate
- Software Development Kit (SDK)
Technical Specifications
| Model Number | 3231 USB |
| Dimensions | 6.2 x 3.4 x 3.2 cm |
| Weight | < 72g (2.5 oz) |
| Finger Size Range | 0.8 – 2.5 cm thickness |
| Power Source | USB Bus Powered |
| Data Output | USB 2.0, 12 Mbit/s |
| Auto Shut-off | 10 seconds after removal |
| Warranty | 1 Year Limited |
Why Choose the Nonin Model 3231 USB?
Proven Accuracy
Over 35 years of pulse oximetry expertise with clinically validated accuracy across all skin tones and perfusion levels.
Seamless Connectivity
USB 2.0 compliance ensures reliable data transmission and easy integration with existing healthcare IT infrastructure.
AI-Ready Platform
Native integration with leading AI healthcare platforms enables predictive analytics and intelligent clinical decision support.
Transform Your Healthcare Practice Today
The Nonin Model 3231 USB Pulse Oximeter represents more than just a medical device—it’s your gateway to the future of AI-powered healthcare. By combining proven clinical accuracy with revolutionary cloud AI integration, this device empowers healthcare providers to deliver personalized, predictive, and proactive patient care.
Contact Information
Nonin Medical, Inc.
Headquarters
13700 1st Avenue North
Plymouth, Minnesota 55441, USA
Phone
+1 (763) 553-9968
(800) 356-8874 (US and Canada)
Fax
+1 (763) 553-7807
info@nonin.com
Website
www.nonin.com
European Representative
MPS Medical Product Service GmbH
Borngasse 20
D-35619 Braunfels, Germany
Technical Support
For technical support, device integration assistance, or AI platform connectivity questions, our expert support team is available to help healthcare organizations maximize their investment in the Nonin Model 3231 USB.
Technical Service Email: technicalservice@nonin.com
International Support: technicalserviceintl@nonin.com
Regulatory Inquiries: regulatory@nonin.com
YOUR AUTOCLAVE CYCLES ON YOUR SMARTPHONE