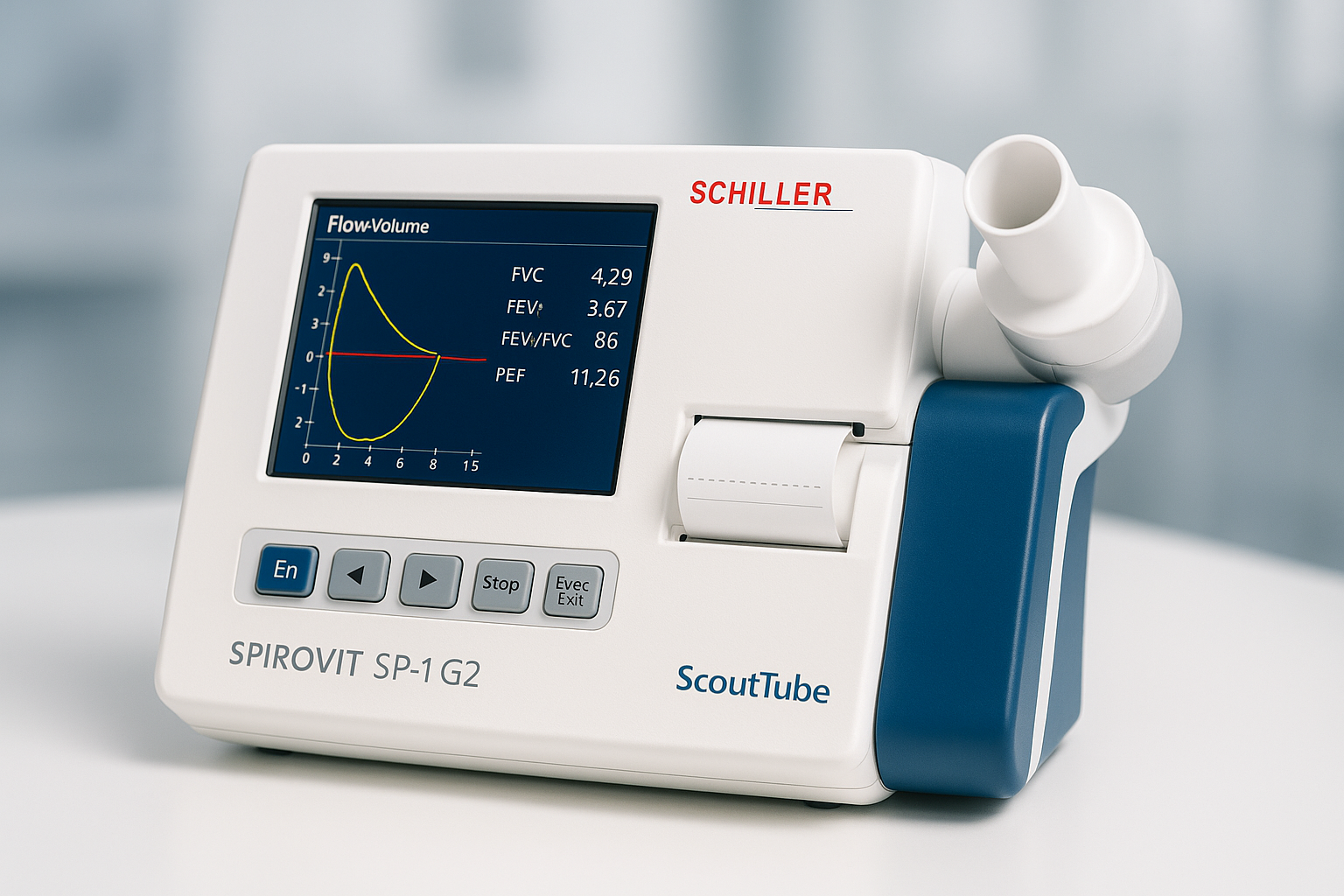
The SCHILLER SPIROVIT SP-1 G2 – Next Generation Spirometer with Advanced AI Integration
The SCHILLER SPIROVIT SP-1 represents a paradigm shift in pulmonary function testing, combining over five decades of Swiss engineering excellence with cutting-edge artificial intelligence and cloud computing technologies. This revolutionary spirometer stands as the pinnacle of respiratory diagnostic equipment, offering healthcare professionals an unprecedented combination of accuracy, reliability, and intelligent data management capabilities.
Built upon the foundation of GANSHORN ultrasound sensor technology, the SPIROVIT SP-1 G2 delivers maintenance-free operation while ensuring the highest standards of measurement precision. The device integrates seamlessly with multiple AI cloud platforms, creating a comprehensive ecosystem for enhanced patient care, predictive analytics, and streamlined clinical workflows that transform traditional spirometry into an intelligent, connected healthcare solution.
SPIROVIT SP-1 G2 with GANSHORN Ultrasound Technology
Advanced Ultrasound Sensors
The proprietary GANSHORN ultrasound sensor technology represents a revolutionary advancement in spirometry measurement systems. Unlike traditional mechanical flow sensors that require frequent calibration and maintenance, these ultrasonic transducers operate without moving parts, ensuring consistent accuracy and eliminating the need for routine maintenance protocols.
The sensors utilize high-frequency ultrasonic waves to measure airflow velocity with unprecedented precision, achieving measurement accuracies that exceed international standards by significant margins. This technology provides drift-free measurements throughout the device’s operational lifetime, ensuring consistent results regardless of environmental conditions or usage frequency.
Maintenance-Free Operation
The SPIROVIT SP-1’s maintenance-free design revolutionizes clinic operations by eliminating the time-consuming calibration procedures and sensor replacements associated with traditional spirometers. Healthcare professionals can focus entirely on patient care without worrying about device maintenance schedules or measurement accuracy degradation.
This innovative approach significantly reduces total cost of ownership while ensuring continuous operational readiness. The system’s self-monitoring capabilities provide real-time performance validation, alerting users to any potential issues before they affect measurement quality.
G2 Generation Enhancements
The second-generation SPIROVIT SP-1 G2 incorporates numerous technological advances based on extensive clinical feedback and emerging healthcare requirements. Enhanced processing power enables real-time AI analysis, while improved connectivity options facilitate seamless integration with hospital information systems and cloud platforms.
Advanced signal processing algorithms provide superior measurement quality even in challenging testing conditions, while the upgraded user interface offers intuitive operation for healthcare professionals of all experience levels. The G2 generation also features enhanced data security protocols and compliance with the latest international medical device standards.
Built-in Thermal Printer
The integrated high-resolution thermal printer provides immediate access to comprehensive test results without requiring external devices or complex setup procedures. This built-in capability ensures that healthcare providers can deliver instant patient feedback and maintain complete documentation standards regardless of their facility’s technological infrastructure.
The printer produces professional-quality reports featuring detailed flow-volume loops, time-volume curves, and comprehensive measurement tables. Advanced formatting options allow customization of report layouts to meet specific clinical requirements and institutional standards.
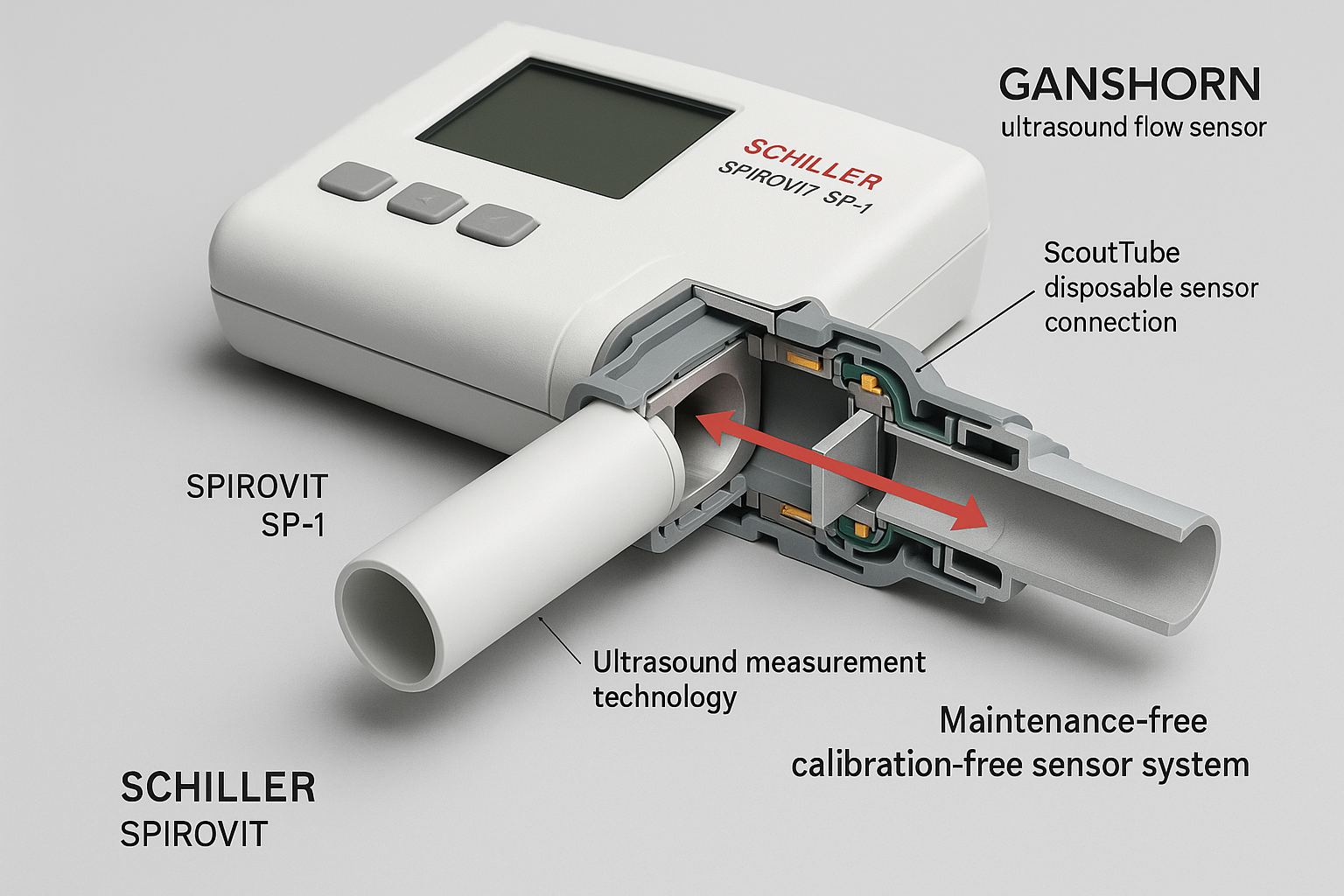
GANSHORN Ultrasound Sensor Technology – Maintenance-Free Precision Measurement System
Technical Specifications
| Specification |
Value |
Standard Compliance |
| Measurement Technology |
GANSHORN Ultrasound Sensors |
ATS/ERS 2019 |
| Flow Range |
±20 L/s |
ATS/ERS Guidelines |
| Volume Range |
0 – 10 L |
ISO 26782:2009 |
| Flow Accuracy |
±2% or ±0.050 L/s |
Exceeds ATS Requirements |
| Volume Accuracy |
±2% or ±0.050 L |
Exceeds ERS Requirements |
| Resistance |
<0.5 cmH2O/L/s |
ATS/ERS Compliant |
| Dead Space |
<50 mL |
Optimized Design |
| Sampling Rate |
200 Hz |
High Resolution |
| Display |
10.1″ Color Touchscreen |
HD Resolution |
| Printer |
Built-in Thermal Printer |
Professional Quality |
| Connectivity |
Wi-Fi, Ethernet, USB, Bluetooth |
Universal Compatibility |
| Power Supply |
100-240V AC, Internal Battery |
Global Compatibility |
| Operating Environment |
15-40°C, 15-95% RH |
Wide Range Operation |
| Dimensions |
380 × 280 × 150 mm |
Compact Desktop Design |
| Weight |
4.2 kg |
Portable Configuration |
The SCHILLER SPIROVIT SP-1 incorporates a comprehensive suite of advanced technical features designed to meet the diverse requirements of modern respiratory medicine. From basic screening applications to complex research protocols, the device provides healthcare professionals with the tools necessary to deliver accurate, reliable, and clinically meaningful pulmonary function assessments across all patient populations.
FVC (Forced Vital Capacity) Testing
Forced Vital Capacity testing represents the cornerstone of spirometric evaluation, and the SPIROVIT SP-1 delivers exceptional performance in this critical measurement domain. The device’s advanced algorithms and ultra-precise sensors ensure accurate capture of the complete expiratory maneuver, from the initial explosive phase through the sustained expiration plateau.
The FVC testing protocol incorporates real-time quality assessment capabilities that guide patients through optimal test performance while providing immediate feedback to healthcare providers regarding test acceptability and reproducibility. Advanced coaching features help patients achieve maximum effort while maintaining proper technique throughout the maneuver.
Key FVC parameters measured include forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, peak expiratory flow (PEF), and forced expiratory flows at various lung volume percentages. These measurements provide comprehensive insight into both obstructive and restrictive pulmonary conditions.
The device’s sophisticated artifact detection algorithms automatically identify and flag potential measurement errors, including coughing, variable effort, premature termination, and hesitation at test initiation. This intelligent quality control ensures that only reliable measurements contribute to the final diagnostic assessment.
Advanced visualization features present real-time flow-volume and volume-time curves during test execution, allowing immediate assessment of test quality and patient effort. The high-resolution display enables detailed examination of curve characteristics that may indicate specific pathological conditions or measurement artifacts.
SVC (Slow Vital Capacity) Testing
Slow Vital Capacity measurements provide essential information about total lung capacity and respiratory muscle function that cannot be obtained through forced maneuvers alone. The SPIROVIT SP-1’s SVC testing capabilities deliver precise measurements of inspiratory and expiratory reserve volumes, tidal volume, and total vital capacity under relaxed breathing conditions.
Inspiratory Capacity Assessment
The system measures inspiratory capacity with exceptional precision, capturing the maximum volume of air a patient can inhale from the functional residual capacity level. This measurement proves particularly valuable in assessing respiratory muscle strength and detecting restrictive pulmonary diseases that may not be apparent during forced breathing maneuvers.
Expiratory Reserve Volume
Precise measurement of expiratory reserve volume provides insight into lung compliance and chest wall mechanics. The SPIROVIT SP-1’s sensitive sensors accurately capture the additional volume that can be exhaled after normal tidal expiration, contributing to comprehensive pulmonary function assessment.
Tidal Volume Monitoring
Continuous tidal volume monitoring during SVC testing provides baseline respiratory function data essential for interpreting other spirometric measurements. The device’s ultra-low resistance design ensures that natural breathing patterns remain unaffected during measurement periods.
MVV (Maximum Voluntary Ventilation) Testing
Maximum Voluntary Ventilation testing evaluates the overall efficiency of the respiratory system by measuring the maximum volume of air a patient can breathe in and out during a specific time period. The SPIROVIT SP-1’s MVV capabilities provide comprehensive assessment of respiratory endurance, coordination, and overall pulmonary system performance.
The device guides patients through the standardized 12-second or 15-second breathing protocol while continuously monitoring breathing pattern, frequency, and volume consistency. Advanced algorithms automatically detect irregular breathing patterns, inadequate effort, or premature fatigue that could compromise test validity.
Intelligent MVV Analysis
The SPIROVIT SP-1’s intelligent analysis system automatically calculates predicted MVV values based on FEV1 measurements and compares actual performance to expected values. This comparison helps identify neuromuscular disorders, respiratory muscle weakness, and coordination problems that may not be apparent through other spirometric tests.
Real-time visual feedback during MVV testing helps patients maintain optimal breathing patterns while providing healthcare providers with immediate assessment of test quality and patient effort. The system’s advanced signal processing capabilities filter breathing irregularities and provide accurate ventilation measurements even in challenging testing conditions.
Maintenance-Free Sensor Technology
The revolutionary maintenance-free sensor design represents one of the SPIROVIT SP-1’s most significant technological advantages over traditional spirometry systems. This innovative approach eliminates the ongoing operational burden associated with sensor calibration, replacement, and verification procedures that typically consume significant clinic resources and create potential sources of measurement error.
Ultrasonic Measurement Principles
The GANSHORN ultrasound sensors operate on the principle of transit-time measurement, where ultrasonic pulses are transmitted across the airflow path in both directions. The time difference between upstream and downstream pulse transmission directly correlates with airflow velocity, providing highly accurate flow measurements without mechanical interference.
This non-contact measurement approach eliminates wear components, drift mechanisms, and environmental sensitivity issues that plague traditional mechanical sensors. The ultrasonic transducers maintain consistent performance characteristics throughout their operational lifetime without requiring periodic calibration or adjustment procedures.
Self-Monitoring Capabilities
Integrated self-monitoring systems continuously verify sensor performance and signal integrity during operation. Advanced diagnostic algorithms automatically detect potential measurement anomalies and alert operators to any conditions that might affect measurement accuracy.
The self-monitoring system includes temperature compensation, signal-to-noise ratio analysis, and transducer performance verification to ensure consistent measurement quality regardless of environmental conditions or usage patterns. This comprehensive monitoring approach provides confidence in measurement reliability without manual intervention.
Integrated Thermal Printer System
The built-in high-resolution thermal printer transforms the SPIROVIT SP-1 into a complete, self-contained spirometry solution that eliminates dependencies on external devices and complex network configurations. This integrated approach ensures immediate availability of professional-quality test reports regardless of the clinical environment’s technological infrastructure.
300 DPI Resolution Fast Print Speed Silent Operation Low Maintenance Standard Paper Custom Layouts
The thermal printer produces comprehensive reports featuring detailed spirometry curves, measurement tables, interpretation guidelines, and customizable header information. Advanced formatting capabilities allow healthcare providers to tailor report layouts to meet specific clinical requirements, institutional standards, and patient documentation needs.
Professional-quality printouts include flow-volume loops with reference curves, time-volume graphs with measurement markers, comprehensive parameter tables with normal value comparisons, and interpretation aids that support clinical decision-making. The printer’s fast operation ensures minimal patient wait times while maintaining superior print quality that meets medical documentation standards.
Advanced Signal Processing
The SPIROVIT SP-1 incorporates sophisticated signal processing algorithms that enhance measurement accuracy and reliability across diverse testing conditions. These advanced processing capabilities enable accurate spirometry measurements even in challenging environments or with patients who have difficulty performing standard breathing maneuvers.
Noise Reduction Algorithms
Advanced digital filtering techniques eliminate environmental noise and measurement artifacts while preserving the integrity of respiratory signals. Multi-stage filtering processes remove electrical interference, vibration artifacts, and other environmental disturbances that could compromise measurement accuracy.
div class=”feature-card”>
Real-Time Quality Assessment
Intelligent quality assessment algorithms continuously evaluate measurement validity during test execution. Real-time analysis identifies potential issues such as air leaks, inadequate patient effort, or procedural errors, providing immediate feedback to ensure optimal test quality.
Artifact Detection
Sophisticated artifact detection systems automatically identify and compensate for common measurement disturbances including coughing, swallowing, and breathing irregularities. These intelligent algorithms ensure that measurement artifacts do not compromise the accuracy of final results.
Temperature Compensation
Automatic temperature and humidity compensation ensures consistent measurement accuracy across varying environmental conditions. The system continuously monitors ambient conditions and applies appropriate corrections to maintain measurement precision regardless of seasonal or facility variations.

Comprehensive AI Cloud Integration Ecosystem – Connecting Healthcare Providers with Advanced Analytics
The SCHILLER SPIROVIT SP-1’s revolutionary AI cloud integration capabilities represent a transformative approach to respiratory healthcare, connecting traditional spirometry with the power of artificial intelligence, machine learning, and cloud-based analytics. This comprehensive integration ecosystem enables healthcare providers to harness the collective intelligence of leading AI platforms, creating unprecedented opportunities for enhanced patient care, predictive diagnostics, and population health management.
By seamlessly connecting with multiple AI cloud platforms, the SPIROVIT SP-1 transforms raw spirometry data into actionable clinical insights, enabling healthcare providers to deliver more personalized, predictive, and preventive respiratory care. This intelligent connectivity represents the future of pulmonary function testing, where individual measurements contribute to broader healthcare intelligence while individual patients benefit from collective learning and advanced analytical capabilities.
Google Health Integration
The SPIROVIT SP-1’s integration with Google Health leverages Google’s advanced machine learning infrastructure and healthcare-specific AI tools to provide enhanced spirometry analysis and population health insights. This partnership combines SCHILLER’s precision measurement technology with Google’s computational power and healthcare expertise to create intelligent respiratory care solutions.
Google Health integration enables advanced features including real-time spirometry interpretation, longitudinal trend analysis, and predictive modeling for respiratory disease progression. The platform’s natural language processing capabilities enhance report generation while machine learning algorithms continuously improve diagnostic accuracy based on global spirometry datasets.
Microsoft Azure Health Integration
Microsoft Azure’s healthcare cloud platform provides the SPIROVIT SP-1 with enterprise-grade security, scalability, and AI capabilities specifically designed for healthcare applications. The Azure integration encompasses comprehensive data management, advanced analytics, and intelligent automation features that enhance clinical workflow efficiency and patient care quality.
Azure Health Bot
Intelligent conversational interfaces that guide patients through spirometry procedures, provide pre-test instructions, and deliver post-test education. The Health Bot integration enhances patient engagement while reducing clinical staff workload through automated interaction management.
Azure Cognitive Services
Advanced image analysis, speech recognition, and natural language processing capabilities that enhance spirometry data interpretation and enable voice-guided testing procedures. These services provide multilingual support and accessibility features for diverse patient populations.
Azure Machine Learning
Comprehensive machine learning platform enabling custom model development for respiratory condition classification, treatment response prediction, and personalized care recommendations. The platform supports continuous learning and model improvement based on real-world clinical data.
Azure IoT Hub
Secure device connectivity and management enabling real-time data streaming, remote monitoring, and automated quality assurance protocols. The IoT Hub integration facilitates comprehensive device management and performance optimization across healthcare networks.
NVIDIA Clara Platform Integration
NVIDIA Clara’s AI-powered healthcare platform brings advanced GPU-accelerated computing and specialized medical AI models to spirometry analysis. This integration enables real-time processing of complex respiratory data, advanced imaging analysis, and sophisticated predictive modeling that enhances diagnostic accuracy and clinical decision-making capabilities.
GPU-Accelerated Analytics
NVIDIA’s powerful GPU computing infrastructure enables real-time processing of complex spirometry datasets, advanced pattern recognition algorithms, and sophisticated machine learning models that would be computationally prohibitive on traditional hardware platforms. This acceleration enables instant analysis and interpretation of spirometry data with unprecedented depth and accuracy.
Clara’s specialized medical AI models provide advanced capabilities including automated quality assessment, intelligent artifact detection, and sophisticated pattern recognition for early disease detection. The platform’s federated learning capabilities enable continuous model improvement while maintaining patient privacy and data security standards.
Amazon Web Services (AWS) Healthcare Integration
Amazon Web Services provides the SPIROVIT SP-1 with comprehensive cloud infrastructure, advanced AI services, and healthcare-specific solutions that enable scalable, secure, and intelligent spirometry data management. AWS integration encompasses data storage, processing, analytics, and machine learning capabilities optimized for healthcare applications.
AWS HealthLake
Comprehensive health data management service that enables secure storage, transformation, and analysis of spirometry data at scale. HealthLake provides FHIR-compliant data management with advanced search and analytics capabilities for population health insights.
Amazon Comprehend Medical
Advanced natural language processing specifically designed for medical text analysis, enabling intelligent interpretation of clinical notes, automated report generation, and enhanced documentation quality for spirometry assessments.
AWS SageMaker
Complete machine learning platform enabling custom model development, training, and deployment for specialized respiratory applications. SageMaker provides tools for building predictive models, classification algorithms, and personalized treatment recommendation systems.
Amazon Textract
Intelligent document analysis service that enables automated extraction and processing of historical spirometry reports, facilitating comprehensive longitudinal analysis and trend identification for improved patient care continuity.
IBM Watson Health Integration
IBM Watson Health brings advanced cognitive computing and AI-powered analytics to spirometry data interpretation and clinical decision support. This integration provides healthcare providers with intelligent insights, evidence-based recommendations, and comprehensive data analytics that enhance the clinical value of spirometry testing.
Viz.ai Integration for Intelligent Imaging
Viz.ai’s specialized medical imaging AI platform enhances the SPIROVIT SP-1’s capabilities by providing intelligent correlation between spirometry data and medical imaging results. This integration enables comprehensive respiratory assessment that combines functional and anatomical insights for enhanced diagnostic accuracy and treatment planning.
The Viz.ai integration facilitates automated correlation of spirometry results with chest imaging findings, providing clinicians with comprehensive respiratory assessments that combine functional measurements with anatomical insights. Advanced algorithms identify patterns and correlations that might not be apparent through individual assessment modalities, enhancing diagnostic confidence and treatment precision.
AIDOC Diagnostic Enhancement
AIDOC’s AI-powered diagnostic platform provides the SPIROVIT SP-1 with advanced pattern recognition and anomaly detection capabilities specifically designed for respiratory conditions. This integration enables early detection of subtle changes in pulmonary function that might indicate developing pathology or treatment response.
Automated Screening
Advanced algorithms automatically screen spirometry results for patterns indicative of various respiratory conditions, providing healthcare providers with intelligent alerts and recommendations for further evaluation or intervention.
Trend Analysis
Sophisticated longitudinal analysis capabilities that identify subtle trends and changes in pulmonary function over time, enabling early intervention and improved patient outcomes through proactive care management.
IDx-DR Diabetic Screening Integration
The integration with IDx-DR’s FDA-approved autonomous AI diagnostic system enables comprehensive screening for diabetic complications that may affect respiratory function. This unique integration provides healthcare providers with enhanced screening capabilities for diabetic patients requiring pulmonary function assessment.
IDx-DR integration facilitates intelligent identification of diabetic patients who may be at increased risk for respiratory complications, enabling targeted screening protocols and enhanced monitoring for this high-risk population. The system’s autonomous diagnostic capabilities complement spirometry assessments with comprehensive risk stratification and clinical decision support.
PathAI Computational Pathology Integration
PathAI’s advanced computational pathology platform enhances the SPIROVIT SP-1’s diagnostic capabilities by providing correlation between spirometry results and histopathological findings. This integration enables comprehensive assessment of respiratory conditions that combines functional testing with advanced tissue analysis insights.
The PathAI integration provides healthcare providers with intelligent correlation between spirometry patterns and potential underlying histopathological changes, enhancing diagnostic accuracy and enabling more targeted treatment approaches. Advanced machine learning algorithms identify subtle patterns that may indicate specific pathological processes affecting respiratory function.

Integrated Clinical Workflow – AI-Enhanced Spirometry in Modern Healthcare Settings
The SCHILLER SPIROVIT SP-1 serves as a comprehensive diagnostic platform for a wide spectrum of clinical applications, from routine screening and occupational health assessments to complex pulmonary disease management and research applications. Its advanced capabilities and intelligent integration features make it an invaluable tool for healthcare providers across diverse medical specialties and clinical settings.
Primary Care and Family Medicine Applications
In primary care settings, the SPIROVIT SP-1 enables comprehensive respiratory screening and early detection of pulmonary conditions that might otherwise go undiagnosed until advanced stages. The device’s user-friendly interface and automated interpretation capabilities make it accessible to healthcare providers with varying levels of spirometry expertise, expanding access to high-quality pulmonary function testing in community healthcare settings.
Preventive Screening Programs
The SPIROVIT SP-1 enables implementation of comprehensive preventive screening programs that identify respiratory conditions in asymptomatic individuals. Early detection capabilities allow for timely intervention and treatment initiation, potentially preventing disease progression and improving long-term patient outcomes. The device’s rapid testing capabilities make population screening programs practical and cost-effective.
Chronic Disease Management
Advanced monitoring capabilities support comprehensive chronic disease management for conditions such as COPD, asthma, and interstitial lung diseases. Longitudinal tracking features enable healthcare providers to monitor disease progression, assess treatment effectiveness, and adjust therapeutic interventions based on objective pulmonary function data.
Pre-operative Assessment
Comprehensive pre-operative pulmonary function assessment enables accurate risk stratification for surgical procedures. The SPIROVIT SP-1’s detailed measurements provide essential information for anesthesia planning, post-operative care protocols, and surgical risk assessment, contributing to improved patient safety and surgical outcomes.
Occupational Health Screening
Specialized occupational health applications enable monitoring of workers exposed to respiratory hazards in industrial environments. Regular screening protocols help identify early signs of occupational lung disease, ensure regulatory compliance, and support workplace safety initiatives through objective pulmonary function monitoring.
Pulmonology and Respiratory Medicine
For pulmonology practices and respiratory medicine specialists, the SPIROVIT SP-1 provides advanced diagnostic capabilities that support complex clinical decision-making and specialized treatment protocols. The device’s sophisticated measurement capabilities and AI-enhanced interpretation features enable detailed assessment of respiratory function across the full spectrum of pulmonary diseases.
Obstructive Lung Disease Management
Comprehensive assessment of obstructive lung diseases including COPD, asthma, and bronchiectasis through detailed flow-volume analysis and response testing. Advanced pattern recognition algorithms help differentiate between different obstructive conditions and assess disease severity with unprecedented accuracy.
Restrictive Disease Evaluation
Precise measurement capabilities enable accurate assessment of restrictive lung diseases including pulmonary fibrosis, chest wall disorders, and neuromuscular conditions. The device’s sensitive volume measurements provide essential data for diagnosis, staging, and monitoring of restrictive pulmonary conditions.
Mixed Pattern Analysis
Advanced analytical capabilities enable identification and characterization of mixed obstructive-restrictive patterns that may indicate complex underlying pathology. Intelligent interpretation algorithms assist in differential diagnosis and treatment planning for patients with multiple respiratory conditions.
Treatment Response Monitoring
Sensitive measurement capabilities enable precise monitoring of treatment responses and disease progression over time. Longitudinal analysis features help optimize therapeutic interventions and assess the effectiveness of various treatment modalities through objective pulmonary function data.
Pediatric Applications
The SPIROVIT SP-1’s advanced capabilities extend to pediatric applications, providing specialized features and protocols designed to accommodate the unique requirements of testing children and adolescents. Child-friendly interfaces and age-appropriate testing protocols ensure accurate measurements while maintaining patient comfort and compliance.
Child-Friendly Testing Environment
Specialized pediatric testing protocols include age-appropriate instructions, visual feedback systems, and gamification elements that encourage proper test performance while maintaining clinical accuracy. The device’s low resistance design and gentle measurement approach ensure patient comfort during testing procedures, improving cooperation and test quality in young patients.
Pediatric-specific reference values and growth-adjusted interpretations provide clinically relevant assessments for developing respiratory systems. The device’s ability to track lung function development over time supports comprehensive pediatric respiratory care and enables early identification of developmental abnormalities or disease processes.
Research and Academic Applications
The SPIROVIT SP-1’s advanced measurement capabilities and comprehensive data management features make it an ideal platform for respiratory research and academic applications. High-precision sensors and detailed data capture capabilities support complex research protocols and contribute to advancing understanding of respiratory physiology and pathophysiology.
Clinical Research Protocols
Advanced data management and export capabilities support complex clinical research protocols requiring detailed spirometry measurements. The device’s high sampling rate and precision measurements provide research-quality data for pharmaceutical studies, intervention trials, and longitudinal population health research.
Educational Applications
Comprehensive educational features support respiratory physiology instruction and clinical training programs. Real-time visualization of respiratory mechanics and detailed measurement explanations provide valuable learning tools for students and residents in respiratory medicine and related specialties.
Population Health Studies
Cloud connectivity and data integration capabilities enable participation in large-scale population health studies and epidemiological research. Standardized measurement protocols and comprehensive data capture support multi-site research initiatives and contribute to advancing respiratory health knowledge.
Quality Assurance Programs
Advanced quality assurance features and automated validation protocols ensure research-quality measurements that meet rigorous scientific standards. Comprehensive audit trails and measurement verification capabilities support regulatory compliance and research data integrity requirements.
Integrated Care and Population Health Benefits
The SPIROVIT SP-1’s intelligent connectivity and cloud integration capabilities enable comprehensive population health management and integrated care delivery models. These advanced features support value-based care initiatives, quality improvement programs, and comprehensive respiratory health management across diverse patient populations.
Population-level data analytics provide insights into respiratory health trends, disease prevalence patterns, and treatment effectiveness across different demographic groups. This information supports public health initiatives, resource allocation decisions, and targeted intervention programs that improve respiratory health outcomes at the community level.
Care Coordination Enhancement
Seamless integration with electronic health records and care coordination platforms enables comprehensive respiratory care management across multiple providers and care settings. Shared data access ensures continuity of care and supports collaborative treatment approaches for complex respiratory conditions.
Quality Metrics and Outcomes
Advanced analytics capabilities support comprehensive quality measurement and outcomes tracking for respiratory care programs. Performance benchmarking and quality improvement initiatives are enhanced through objective, standardized spirometry measurements and longitudinal outcome analysis.
Telehealth and Remote Monitoring
The SPIROVIT SP-1’s advanced connectivity features enable comprehensive telehealth applications and remote monitoring capabilities that extend respiratory care beyond traditional clinical settings. These innovative features support distributed care delivery models and enable continuous monitoring of respiratory health in home and community settings.
Remote monitoring capabilities enable real-time assessment of respiratory function changes, early detection of disease exacerbations, and proactive intervention before clinical deterioration occurs. This approach improves patient outcomes while reducing healthcare costs through prevention of emergency department visits and hospitalizations.
Remote Data Access Real-time Monitoring Alert Systems Virtual Consultations Home Testing Cloud Analytics
The SCHILLER SPIROVIT SP-1 represents the culmination of over five decades of Swiss engineering excellence and unwavering commitment to advancing medical technology. Since its founding in 1974, SCHILLER has established itself as a global leader in medical device manufacturing, combining traditional Swiss craftsmanship with cutting-edge innovation to create products that set industry standards for quality, reliability, and clinical performance.
Foundation and Early Innovation (1974-1990)
Founded in the heart of Switzerland’s precision manufacturing region, SCHILLER began its journey with a clear mission: to develop medical devices that would enhance patient care through superior technology and uncompromising quality. The company’s early focus on cardiovascular monitoring and diagnostic equipment established the foundation for its reputation as a manufacturer of precision medical instruments.
The company’s founding principles emphasized precision engineering, innovative design, and clinical excellence—values that continue to guide product development and manufacturing processes today. Early investments in research and development established SCHILLER as a technology leader, with groundbreaking innovations in ECG monitoring, defibrillation, and patient monitoring systems.
Throughout the 1980s, SCHILLER expanded its product portfolio while maintaining strict quality standards that became synonymous with the SCHILLER brand. The company’s commitment to Swiss manufacturing excellence and precision engineering created a strong foundation for future growth and technological advancement.
Strategic partnerships with leading medical institutions and research organizations enabled SCHILLER to develop products that addressed real clinical needs while incorporating the latest technological advances. This collaborative approach to innovation became a hallmark of the SCHILLER development process and continues to influence product design today.
The establishment of rigorous quality management systems and international certification processes during this period laid the groundwork for SCHILLER’s global expansion and reputation for manufacturing excellence that extends across all product lines and geographic markets.
Global Expansion and Technology Leadership (1990-2010)
The 1990s marked a period of significant expansion for SCHILLER, with the company establishing international manufacturing facilities and expanding its presence in global markets. This growth was accompanied by substantial investments in research and development that resulted in groundbreaking innovations across multiple medical device categories.
International Manufacturing Excellence
SCHILLER established world-class manufacturing facilities across multiple continents while maintaining Swiss quality standards and engineering excellence. These facilities incorporate advanced automation, precision manufacturing techniques, and comprehensive quality assurance protocols that ensure consistent product quality regardless of production location.
Technology Innovation Centers
The establishment of dedicated research and development centers enabled SCHILLER to accelerate innovation and maintain technology leadership across multiple product categories. These centers focus on emerging technologies, clinical research, and collaborative development projects with leading medical institutions.
Regulatory Excellence
SCHILLER developed comprehensive regulatory expertise and quality management systems that enable global market access while maintaining the highest standards for safety and effectiveness. This regulatory excellence supports rapid product introduction and ensures compliance with international medical device standards.
Clinical Partnerships
Strategic partnerships with leading hospitals, research institutions, and clinical specialists provide ongoing insights into clinical needs and emerging trends. These collaborations ensure that SCHILLER products address real-world healthcare challenges while incorporating the latest clinical evidence and best practices.
Digital Transformation and AI Integration (2010-Present)
The past decade has seen SCHILLER embrace digital transformation and artificial intelligence integration across its entire product portfolio. This strategic evolution reflects the company’s commitment to staying at the forefront of medical technology while maintaining its core values of precision, reliability, and clinical excellence.
Innovation Through Digital Excellence
SCHILLER’s digital transformation initiative encompasses cloud connectivity, artificial intelligence integration, and advanced data analytics capabilities that transform traditional medical devices into intelligent healthcare platforms. This approach creates new opportunities for enhanced patient care while maintaining the reliability and precision that defines the SCHILLER brand.
The development of the SPIROVIT SP-1 represents the pinnacle of this digital transformation, combining SCHILLER’s traditional engineering excellence with advanced AI capabilities and cloud integration. This innovative approach creates unprecedented opportunities for enhanced patient care while maintaining the precision and reliability that healthcare providers expect from SCHILLER products.
Swiss Engineering Excellence
Swiss engineering excellence remains at the core of every SCHILLER product, including the SPIROVIT SP-1. This commitment to precision manufacturing and quality excellence is reflected in every aspect of product design, from initial concept development through final production and ongoing support services.
Precision Manufacturing
SCHILLER’s Swiss manufacturing facilities employ state-of-the-art precision manufacturing techniques and quality control processes that ensure consistent product quality and performance. Advanced metrology systems and automated quality inspection processes maintain manufacturing tolerances that exceed industry standards for medical devices.
Quality Assurance Excellence
Comprehensive quality management systems encompass every aspect of product development and manufacturing, from initial design validation through final product release. These systems ensure that every SCHILLER product meets or exceeds international quality standards while maintaining the reliability and performance characteristics that define Swiss engineering excellence.
Innovation and Tradition
The combination of traditional Swiss craftsmanship with cutting-edge innovation creates products that offer both reliability and advanced functionality. This unique approach enables SCHILLER to develop products that incorporate the latest technological advances while maintaining the durability and precision that healthcare providers depend upon.
Continuous Improvement
SCHILLER’s commitment to continuous improvement drives ongoing product development and manufacturing process optimization. Regular assessment of customer feedback, clinical outcomes, and technological advances ensures that SCHILLER products continue to evolve and improve over time while maintaining core quality characteristics.
Global Impact and Recognition
Over five decades of innovation and excellence have established SCHILLER as a trusted partner for healthcare providers worldwide. The company’s products are utilized in hospitals, clinics, research institutions, and emergency services across more than 190 countries, contributing to improved patient outcomes and enhanced healthcare delivery globally.
190+ Countries 50+ Years Innovation Swiss Quality Global Manufacturing ISO Certified FDA Approved CE Marked Research Partnerships
Industry recognition and awards acknowledge SCHILLER’s contributions to medical technology advancement and its commitment to quality excellence. These accolades reflect the company’s ongoing dedication to innovation and its role in advancing healthcare through superior medical device technology.
Future Vision and Commitment
SCHILLER’s vision for the future encompasses continued innovation in medical technology while maintaining unwavering commitment to quality, precision, and clinical excellence. The company’s strategic focus on artificial intelligence, digital health, and personalized medicine creates opportunities for transformative healthcare solutions that improve patient outcomes while enhancing clinical efficiency.
The SPIROVIT SP-1 represents just the beginning of SCHILLER’s next generation of intelligent medical devices. Future developments will continue to integrate advanced AI capabilities, cloud connectivity, and personalized healthcare features while maintaining the Swiss engineering excellence and clinical reliability that define the SCHILLER brand.
The ScoutTube disposable sensor system represents a revolutionary approach to spirometry hygiene and infection control, providing healthcare facilities with unprecedented protection against cross-contamination while maintaining measurement accuracy and clinical reliability. This innovative system addresses critical infection control requirements in modern healthcare environments where patient safety and staff protection are paramount concerns.
Revolutionary Hygiene Technology
The ScoutTube system incorporates advanced materials science and precision engineering to create disposable sensors that maintain the accuracy and reliability of permanent sensor systems while providing complete protection against biological contamination. Each ScoutTube sensor is manufactured to exact specifications using medical-grade materials that ensure consistent performance across all testing applications.
Single-Use Protection
Each ScoutTube sensor provides complete single-use protection, eliminating any possibility of cross-contamination between patients. This approach provides absolute assurance of infection control while maintaining the convenience and efficiency of rapid patient testing in high-volume clinical environments.
Medical-Grade Materials
Advanced biocompatible materials ensure patient safety while maintaining measurement accuracy and sensor reliability. The ScoutTube system utilizes materials that meet the highest medical device standards for safety, biocompatibility, and environmental stability.
Easy Integration
Seamless integration with the SPIROVIT SP-1 system ensures that hygiene enhancement does not compromise measurement accuracy or clinical workflow efficiency. The ScoutTube system maintains all advanced measurement capabilities while providing superior infection control protection.
Cost-Effective Solution
The ScoutTube system provides cost-effective infection control that eliminates the need for complex cleaning protocols, expensive sterilization equipment, and time-consuming disinfection procedures. This approach reduces operational costs while enhancing patient and staff safety.
Enhanced Patient Safety
Patient safety represents the primary driver behind ScoutTube system development, with comprehensive protection against respiratory pathogens, bacterial contamination, and viral transmission. This advanced protection system provides peace of mind for patients and healthcare providers while maintaining the clinical accuracy necessary for reliable diagnostic assessments.
Complete Contamination Prevention
The ScoutTube system provides absolute protection against contamination from respiratory pathogens, including viruses, bacteria, and other microorganisms that may be present in patient breath samples. This comprehensive protection ensures that each patient receives a completely sterile testing environment while protecting subsequent patients from any potential exposure.
Clinical Workflow Advantages
Beyond safety considerations, the ScoutTube system provides significant clinical workflow advantages that enhance operational efficiency and reduce administrative burden in healthcare facilities. These advantages make high-volume spirometry testing more practical while maintaining the highest standards for patient care and safety.
Simplified Procedures
Elimination of complex cleaning and sterilization procedures reduces testing time and simplifies clinical protocols. Healthcare staff can focus on patient care rather than device maintenance, improving overall efficiency and patient satisfaction while reducing operational complexity.
Reduced Downtime
The ScoutTube system eliminates device downtime associated with cleaning and sterilization procedures, enabling continuous testing capabilities in high-volume clinical environments. This improved availability supports better patient scheduling and reduced waiting times.
Regulatory Compliance
The ScoutTube system supports compliance with evolving infection control regulations and guidelines while providing documentation and traceability features that support quality assurance and regulatory reporting requirements.
Quality Assurance
Each ScoutTube sensor undergoes rigorous quality testing and validation to ensure consistent performance characteristics. This comprehensive quality assurance process provides confidence in measurement reliability while maintaining superior hygiene standards.
The SCHILLER SPIROVIT SP-1’s comprehensive pre/post bronchodilator testing capabilities provide healthcare professionals with essential tools for differential diagnosis, treatment optimization, and longitudinal monitoring of respiratory conditions. These advanced testing protocols enable precise assessment of airway responsiveness and treatment effectiveness across diverse patient populations and clinical scenarios.
Comprehensive Bronchodilator Response Testing
Bronchodilator response testing represents a critical diagnostic tool for differentiating between reversible and irreversible airway obstruction, guiding treatment decisions, and monitoring therapeutic effectiveness. The SPIROVIT SP-1’s advanced capabilities enable standardized testing protocols that provide reliable, reproducible results essential for clinical decision-making.
Standardized Protocols
Built-in standardized testing protocols ensure consistent methodology and reliable results across different operators and testing sessions. These protocols incorporate international guidelines and best practices for bronchodilator response assessment, ensuring clinical validity and reproducibility of results.
Automated Analysis
Intelligent analysis algorithms automatically calculate bronchodilator response parameters and provide clinical interpretation guidance. This automated approach reduces variability and ensures accurate assessment of treatment response according to established clinical criteria.
Timing Management
Integrated timing systems ensure appropriate intervals between pre- and post-bronchodilator measurements, optimizing clinical protocols and ensuring valid results. Automatic reminders and scheduling features support consistent testing procedures and reliable outcomes.
Comparative Analysis
Advanced comparative analysis features provide detailed assessment of changes between pre- and post-bronchodilator measurements, including statistical significance testing and clinical interpretation guidance. These features support evidence-based clinical decision-making and treatment optimization.
Clinical Applications and Diagnostic Value
Pre/post bronchodilator testing provides essential diagnostic information for multiple respiratory conditions, enabling healthcare providers to differentiate between various forms of airway obstruction and assess the potential for therapeutic intervention. This testing approach is particularly valuable for asthma diagnosis, COPD assessment, and treatment optimization across diverse patient populations.
Asthma diagnosis benefits significantly from bronchodilator response testing, as reversible airway obstruction represents a key diagnostic criterion. The SPIROVIT SP-1’s precise measurements enable detection of even subtle improvements in airway function following bronchodilator administration, supporting accurate diagnosis and appropriate treatment initiation.
COPD assessment utilizes bronchodilator response testing to evaluate the degree of reversible airway obstruction present in addition to fixed obstruction. This information guides treatment decisions regarding bronchodilator therapy effectiveness and helps optimize medication selection and dosing protocols.
Mixed respiratory conditions may exhibit complex patterns of bronchodilator response that require sophisticated analysis and interpretation. The SPIROVIT SP-1’s advanced analytical capabilities help identify these complex patterns and provide clinical guidance for managing patients with multiple respiratory conditions.
Treatment monitoring applications utilize serial bronchodilator response testing to assess therapeutic effectiveness over time and guide medication adjustments. This approach enables optimization of treatment protocols based on objective measurement data rather than subjective symptom reporting alone.

Professional Spirometry Report with Pre/Post Bronchodilator Analysis and Clinical Interpretation
SCHILLER SPIROVIT SP-1 G2
$24,995
Complete System with AI Cloud Integration
Request Quote
Complete System Inclusions
Core System Components
- SPIROVIT SP-1 G2 Main Unit
- GANSHORN Ultrasound Sensors
- Built-in Thermal Printer
- 10.1″ HD Color Touchscreen
- Internal Rechargeable Battery
- Universal Power Adapter
Software and Integration
- Advanced Spirometry Software
- AI Cloud Platform Access
- Multi-Platform Integration
- Electronic Health Record Connectivity
- Remote Monitoring Capabilities
- Automatic Software Updates
Support and Training
- Comprehensive Installation Service
- Clinical Staff Training Program
- Technical Support Access
- Online Training Resources
- Documentation and Manuals
- Warranty Coverage
Optional Accessories
- ScoutTube Disposable Sensors
- Mobile Cart System
- Additional Printer Paper
- Extended Warranty Options
- Advanced Training Programs
- Custom Integration Services
Financing and Leasing Options
SCHILLER offers comprehensive financing and leasing options designed to make advanced spirometry technology accessible to healthcare facilities of all sizes. These flexible payment programs enable implementation of cutting-edge respiratory diagnostic capabilities while managing capital expenditure requirements and cash flow considerations.
Lease-to-Own Programs
Flexible lease-to-own programs enable healthcare facilities to implement advanced spirometry technology with manageable monthly payments. These programs provide immediate access to the latest technology while building equity toward eventual ownership.
Equipment Financing
Traditional equipment financing options provide competitive interest rates and flexible terms tailored to healthcare facility requirements. These programs enable acquisition of advanced technology while preserving working capital for other operational needs.
Trade-In Programs
Existing spirometry equipment trade-in programs provide value credit toward new system acquisition while ensuring proper disposal of legacy equipment. These programs reduce total investment cost while supporting technology upgrade initiatives.
Volume Discounts
Multi-unit purchases and healthcare system-wide implementations qualify for substantial volume discounts and enhanced support services. These programs support standardization initiatives while providing cost advantages for large-scale deployments.
Return on Investment Analysis
Investment in the SCHILLER SPIROVIT SP-1 provides substantial return through enhanced clinical capabilities, operational efficiency improvements, and expanded service offerings. The system’s advanced features and intelligent integration capabilities create multiple opportunities for revenue enhancement and cost reduction that justify the initial investment.
Total Cost of Ownership Advantages
The SPIROVIT SP-1’s maintenance-free design, integrated printing capabilities, and comprehensive software suite significantly reduce total cost of ownership compared to traditional spirometry systems. These advantages include elimination of calibration costs, reduced maintenance requirements, and comprehensive functionality that eliminates the need for additional equipment purchases.





