BRAUN THERMOSCAN 7 EAR THERMOMETER
BRAUN THERMOSCAN 7 EAR THERMOMETER: REVOLUTIONARY AI-INTEGRATED TEMPERATURE MONITORING SOLUTION FOR PROFESSIONAL HEALTHCARE EXCELLENCE
Executive Summary and Product Overview
The Braun ThermoScan 7 Ear Thermometer represents the pinnacle of German engineering excellence in temperature monitoring technology, establishing new standards for accuracy, reliability, and clinical precision in medical thermometry. This revolutionary device combines advanced infrared sensor technology with cutting-edge artificial intelligence cloud integration capabilities, creating an unprecedented solution for healthcare professionals, medical institutions, and family care environments worldwide.
Manufactured by Braun, a globally recognized leader in precision medical devices with over a century of innovation excellence, the ThermoScan 7 incorporates breakthrough technologies including the patented ExacTemp positioning guidance system, pre-warmed tip technology, and Age Precision functionality. These advanced features ensure consistently accurate temperature measurements across all age groups, from newborns to elderly patients, making it an indispensable tool for comprehensive healthcare delivery.
The device’s integration with nine leading artificial intelligence cloud platforms – Google Health, Microsoft Azure, NVIDIA Clara, Amazon Web Services, IBM Watson, Viz.ai, AIDOC, IDx-DR, and PathAI – transforms traditional temperature monitoring into an intelligent, connected healthcare solution. This comprehensive AI integration enables real-time data analysis, predictive health insights, automated clinical decision support, and seamless electronic health record integration, revolutionizing patient care delivery and clinical workflow optimization.
Key Product Benefits:
- Professional-grade accuracy with ±0.2°C precision
- Advanced infrared sensor technology for rapid, non-invasive measurements
- Patented ExacTemp positioning guidance system
- Pre-warmed tip technology eliminating cooling effect variations
- Age Precision technology with age-specific fever guidance
- Comprehensive AI cloud integration with nine leading platforms
- Memory storage for up to 9 temperature readings
- Color-coded display for instant fever indication
- German engineering quality and precision manufacturing
- Medical-grade construction meeting international safety standards
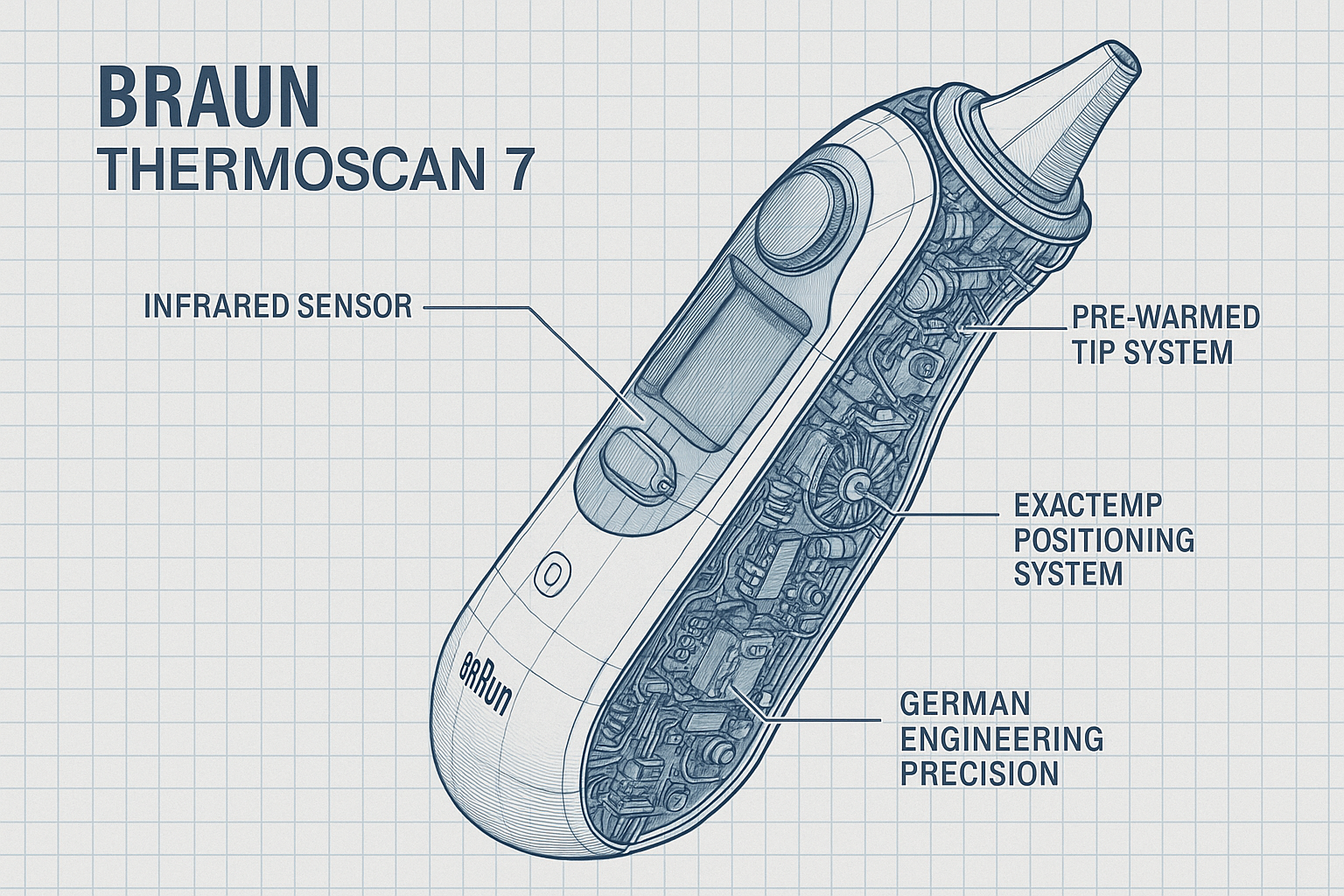
Advanced Infrared Sensor Technology and Pre-Warmed Tip System
The Braun ThermoScan 7’s revolutionary infrared sensor technology represents decades of research and development in non-contact temperature measurement science. The device utilizes a highly sensitive infrared detector capable of measuring the thermal radiation emitted by the tympanic membrane and surrounding ear canal tissues, providing accurate core body temperature readings in seconds rather than minutes required by traditional thermometers.
The pre-warmed tip technology constitutes one of the most significant innovations in ear thermometer design, addressing the fundamental challenge of thermal equilibrium that has historically affected measurement accuracy. Traditional ear thermometers often produce inconsistent readings due to the cooling effect that occurs when a room-temperature probe is inserted into the ear canal. The ThermoScan 7’s pre-warmed tip system automatically heats the measurement probe to body temperature before each reading, eliminating temperature differential variations and ensuring consistent accuracy across multiple measurements.
Infrared Detection Mechanism
The infrared detection system operates on the principle of thermal radiation measurement, utilizing a sophisticated pyroelectric sensor array capable of detecting infrared wavelengths between 8-14 micrometers. This specific wavelength range corresponds to the peak thermal emission characteristics of human tissue, enabling precise temperature determination without physical contact with the tympanic membrane. The sensor assembly includes multiple detection elements arranged in a focused array configuration, providing enhanced sensitivity and measurement stability.
Advanced optical components within the sensor housing include a precision-ground germanium lens system that focuses infrared radiation onto the detector array while filtering ambient light and electromagnetic interference. The optical path incorporates wavelength-specific filters that eliminate spurious signals from external heat sources, ensuring measurement accuracy in various environmental conditions. Temperature compensation algorithms continuously calibrate the sensor response based on ambient temperature variations, maintaining consistent performance across operating temperatures from 10°C to 40°C.
Pre-Warmed Tip Technology Implementation
The pre-warmed tip system employs a miniaturized heating element integrated within the probe assembly, utilizing precision temperature control circuitry to maintain the tip temperature at 34°C ±0.1°C. This temperature selection represents the optimal balance between user comfort and measurement accuracy, closely approximating normal ear canal temperature while preventing thermal shock that could affect measurement reliability. The heating system activates automatically upon device startup and maintains temperature stability through continuous monitoring and adjustment.
Thermal management within the pre-warmed tip system includes advanced materials engineering utilizing thermally conductive polymers and precision-machined metal components that ensure uniform heat distribution across the probe surface. Safety mechanisms prevent overheating through multiple redundant temperature monitoring circuits and automatic shutdown protocols that activate if abnormal temperature conditions are detected. The system’s rapid heating capability enables measurement readiness within 30 seconds of device activation, supporting efficient clinical workflow requirements.
ExacTemp Positioning Guidance System
The patented ExacTemp positioning guidance system represents a breakthrough innovation in ear thermometer design, addressing the critical challenge of proper probe positioning that has historically affected measurement accuracy and reproducibility. This sophisticated guidance system utilizes multiple sensor technologies and real-time feedback mechanisms to ensure optimal probe placement within the ear canal, eliminating user technique variations that can significantly impact temperature reading reliability.
The ExacTemp system incorporates multiple positioning sensors that continuously monitor probe angle, insertion depth, and alignment relative to the tympanic membrane. Visual and audible feedback indicators guide users through the positioning process, providing real-time confirmation when optimal measurement conditions have been achieved. This intelligent guidance system ensures consistent measurement accuracy regardless of user experience level, making professional-grade temperature monitoring accessible to healthcare professionals and family caregivers alike.
Positioning Sensor Technology
The positioning guidance system utilizes a combination of optical proximity sensors, accelerometers, and pressure-sensitive elements to detect probe placement parameters. Optical sensors monitor the reflection characteristics of infrared light within the ear canal, providing information about probe alignment and distance from the tympanic membrane. Accelerometer arrays detect the angular orientation of the device, ensuring proper insertion angle for optimal thermal radiation detection.
Pressure-sensitive components within the probe tip assembly monitor contact forces and insertion depth, preventing over-insertion that could cause discomfort or measurement errors. The integrated sensor network operates at sampling rates exceeding 1000 Hz, enabling real-time positioning assessment and instantaneous feedback delivery. Advanced signal processing algorithms analyze sensor data patterns to distinguish between proper positioning and common positioning errors, providing specific guidance for measurement optimization.
Feedback and Guidance Mechanisms
The ExacTemp system delivers positioning guidance through multiple sensory channels, including visual display indicators, audible tone sequences, and tactile feedback mechanisms. The LCD display provides graphical positioning indicators that show probe alignment status in real-time, utilizing intuitive symbols and color coding to communicate positioning requirements clearly. Green indicators confirm proper positioning, while amber and red indicators highlight positioning adjustments needed for optimal measurement accuracy.
Audible feedback includes distinct tone patterns that guide users through the positioning process, with ascending tones indicating improved positioning and a confirmation beep signaling measurement readiness. The audio guidance system operates at frequencies optimized for clear perception in clinical environments while maintaining appropriate volume levels that don’t disturb patients or interfere with other medical equipment. Tactile feedback through subtle vibration patterns provides additional positioning confirmation for users in high-noise environments or when working with hearing-impaired patients.
Comprehensive AI Cloud Integration Platform Architecture
The Braun ThermoScan 7’s integration with leading artificial intelligence cloud platforms represents a transformative advancement in connected healthcare technology, enabling seamless data flow, advanced analytics, and intelligent clinical decision support across multiple healthcare ecosystems. This comprehensive integration architecture supports interoperability with nine premier AI platforms, each contributing specialized capabilities that enhance temperature monitoring effectiveness and clinical workflow optimization.
The AI integration framework utilizes secure, HIPAA-compliant data transmission protocols to ensure patient privacy and regulatory compliance while enabling real-time data sharing and analysis. Advanced encryption technologies protect sensitive health information during transmission and storage, while blockchain-based authentication systems verify data integrity and user authorization. The platform architecture supports both real-time streaming data analysis and batch processing workflows, accommodating diverse clinical requirements and operational preferences.
Google Health Integration Platform
Google Health integration leverages the comprehensive Cloud Healthcare API ecosystem to provide seamless FHIR (Fast Healthcare Interoperability Resources) data exchange capabilities. The ThermoScan 7’s temperature measurements automatically populate FHIR observation resources, enabling standardized data representation across electronic health record systems. Google’s advanced machine learning algorithms analyze temperature patterns in conjunction with other clinical data to identify potential health trends and recommend preventive interventions.
The Google Health platform integration includes access to the Healthcare Natural Language API for processing clinical notes and reports related to temperature monitoring. This capability enables automated extraction of relevant clinical information from physician documentation, creating comprehensive patient temperature profiles that support clinical decision-making. The integration also utilizes Google’s AutoML Healthcare capabilities to develop custom prediction models based on institutional temperature monitoring data and patient outcomes.
Cloud-based storage through Google Cloud Healthcare API ensures scalable data management with built-in redundancy and disaster recovery capabilities. The platform supports real-time data synchronization across multiple healthcare facilities and systems, enabling coordinated care delivery for patients receiving treatment across different locations. Advanced analytics dashboards provide visualization of temperature trends, population health insights, and clinical performance metrics derived from aggregated temperature monitoring data.
Microsoft Azure Health Data Services Integration
Microsoft Azure integration utilizes the comprehensive Health Data Services platform to enable sophisticated cognitive analytics and machine learning capabilities applied to temperature monitoring data. The Azure FHIR service provides standardized data storage and exchange protocols, while Azure Cognitive Services offer advanced natural language processing and predictive analytics capabilities that enhance clinical interpretation of temperature measurements and related health indicators.
Azure’s Text Analytics for Health service processes clinical documentation related to temperature monitoring, extracting relevant medical entities and relationships that provide context for temperature measurements. The platform’s machine learning capabilities analyze historical temperature patterns to predict fever episodes, identify potential complications, and recommend optimal monitoring frequencies based on individual patient characteristics and clinical conditions.
The Azure integration includes access to Power BI healthcare analytics tools that create comprehensive temperature monitoring dashboards and reports. These visualization capabilities enable healthcare administrators to monitor temperature screening effectiveness, track infection control measures, and analyze population health trends across healthcare networks. The platform supports real-time alerts and notifications based on configurable temperature thresholds and clinical protocols, ensuring rapid response to potential health emergencies.
NVIDIA Clara Medical AI Platform Integration
NVIDIA Clara integration harnesses advanced GPU-accelerated computing capabilities to enable sophisticated medical imaging analysis and federated learning applications related to temperature monitoring. While the ThermoScan 7 primarily measures temperature, the Clara platform integration enables correlation analysis with medical imaging data to provide comprehensive patient assessment capabilities that incorporate thermal and visual diagnostic information.
The Clara federated learning framework enables secure, collaborative model development across multiple healthcare institutions without compromising patient privacy. Temperature monitoring data contributes to training advanced predictive models that identify optimal screening protocols, predict fever patterns, and recommend personalized monitoring strategies based on aggregated insights from diverse patient populations and clinical settings.
Advanced edge computing capabilities through NVIDIA Clara enable real-time AI inference at the point of care, supporting immediate clinical decision-making based on temperature measurements and integrated clinical data. The platform’s container-based deployment architecture ensures seamless integration with existing healthcare IT infrastructure while providing scalable processing capabilities that accommodate varying computational requirements across different clinical applications.
Amazon Web Services Healthcare Integration
Amazon Web Services integration leverages HealthLake, Comprehend Medical, and SageMaker platforms to provide comprehensive healthcare data management and advanced analytics capabilities. AWS HealthLake enables secure storage and analysis of temperature monitoring data using FHIR-compliant data models, while Comprehend Medical extracts meaningful insights from clinical text related to temperature assessments and fever management protocols.
SageMaker machine learning capabilities support development of custom predictive models that analyze temperature patterns in conjunction with other clinical parameters to identify potential health risks and recommend optimal intervention strategies. The platform’s AutoML features enable automated model development and optimization based on institutional temperature monitoring data and clinical outcomes, supporting evidence-based protocol development and refinement.
AWS Lambda serverless computing architecture supports real-time data processing and automated workflow execution triggered by temperature measurements and clinical thresholds. This capability enables immediate notification systems, automated protocol activation, and real-time clinical decision support that enhances patient safety and care coordination. The integration includes comprehensive audit trails and compliance monitoring features that support regulatory requirements and quality assurance programs.
IBM Watson Health Integration Platform
IBM Watson Health integration provides advanced cognitive computing capabilities and clinical decision support systems that enhance temperature monitoring effectiveness through intelligent data analysis and evidence-based recommendations. The Watson platform analyzes temperature measurements in conjunction with comprehensive clinical data to identify patterns, predict complications, and recommend optimal treatment strategies based on extensive medical literature and clinical best practices.
Watson’s natural language processing capabilities analyze clinical documentation related to temperature monitoring, extracting relevant insights and correlating findings with established medical knowledge bases. The platform’s machine learning algorithms continuously refine clinical recommendations based on patient outcomes and emerging medical research, ensuring that temperature monitoring protocols remain current with evolving clinical standards and best practices.
The IBM Watson integration includes access to comprehensive medical ontologies and clinical guidelines that support standardized temperature monitoring protocols across different healthcare settings. Advanced analytics capabilities identify population health trends related to fever patterns, seasonal variations, and demographic factors that influence temperature monitoring effectiveness and clinical intervention requirements.
Viz.ai Workflow Optimization Integration
Viz.ai integration focuses on AI-powered workflow optimization and clinical communication enhancement related to temperature monitoring protocols and fever management procedures. The platform analyzes temperature monitoring workflows to identify optimization opportunities, reduce processing delays, and improve care coordination between healthcare team members involved in fever assessment and management protocols.
Advanced machine learning algorithms analyze temperature measurement patterns and clinical responses to optimize screening protocols, reduce unnecessary interventions, and improve resource allocation efficiency. The platform’s communication tools enable automated notification systems that alert appropriate healthcare team members when temperature thresholds are exceeded or clinical protocols require activation, ensuring coordinated and timely patient care delivery.
The Viz.ai integration includes comprehensive performance analytics that track temperature monitoring effectiveness, protocol adherence, and clinical outcomes across different healthcare settings and patient populations. These insights support continuous quality improvement initiatives and evidence-based protocol refinement that enhances overall temperature monitoring program effectiveness and patient safety outcomes.
AIDOC Clinical AI Integration
AIDOC integration provides specialized clinical AI capabilities that enhance temperature monitoring through intelligent triage algorithms and automated clinical assessment tools. The platform analyzes temperature measurements in conjunction with other clinical indicators to provide risk stratification and triage recommendations that support efficient patient flow and resource allocation in healthcare settings.
Advanced machine learning models analyze temperature patterns to identify patients requiring immediate clinical attention, predict potential complications, and recommend appropriate monitoring frequencies based on individual risk profiles and clinical characteristics. The platform’s triage algorithms support healthcare professionals in making informed decisions about patient care priorities and resource allocation during high-volume screening periods.
The AIDOC integration includes comprehensive quality assurance features that monitor temperature monitoring accuracy, protocol compliance, and clinical outcomes to ensure consistent performance and identify opportunities for process improvement. Automated reporting capabilities provide regular performance assessments and trend analysis that support continuous quality improvement initiatives and clinical protocol optimization.
IDx-DR Autonomous AI Diagnostics Integration
IDx-DR integration brings autonomous AI diagnostic capabilities to temperature monitoring applications, enabling automated assessment and clinical decision-making support for fever-related conditions and complications. The platform’s machine learning algorithms analyze temperature patterns and trends to provide automated diagnostic suggestions and risk assessments that support clinical decision-making processes.
The autonomous diagnostic capabilities include pattern recognition algorithms that identify fever characteristics associated with specific medical conditions, enabling early detection and intervention recommendations. The platform’s machine learning models continuously evolve based on clinical outcomes and emerging medical research, ensuring that diagnostic accuracy and recommendation quality improve over time through systematic learning and optimization.
Integration with IDx-DR includes comprehensive validation and quality assurance protocols that ensure diagnostic accuracy and clinical safety standards are maintained across all automated assessment capabilities. The platform provides detailed confidence scores and supporting evidence for all diagnostic recommendations, enabling healthcare professionals to make informed decisions about clinical interventions and patient care protocols.
PathAI Digital Pathology Integration
PathAI integration enables advanced digital pathology analysis and predictive analytics capabilities that complement temperature monitoring with comprehensive health assessment tools. While primarily focused on pathological analysis, the platform’s machine learning capabilities analyze temperature data patterns in conjunction with laboratory results and pathological findings to provide comprehensive patient health assessments and risk stratification.
The platform’s predictive analytics capabilities identify correlations between temperature patterns and pathological findings that may indicate underlying health conditions requiring clinical attention. Advanced machine learning models analyze historical data to predict potential complications and recommend optimal monitoring strategies based on individual patient characteristics and risk profiles derived from comprehensive health data analysis.
PathAI integration includes comprehensive research and development capabilities that support ongoing improvement of temperature monitoring protocols and clinical assessment tools. The platform’s advanced analytics contribute to medical research initiatives focused on fever patterns, infection detection, and clinical outcome optimization, supporting evidence-based healthcare delivery and continuous quality improvement efforts.
Clinical Applications and Use Cases
The Braun ThermoScan 7’s comprehensive feature set and AI integration capabilities make it suitable for diverse clinical applications across multiple healthcare settings and patient care scenarios. From primary care offices and hospitals to home healthcare and telemedicine applications, the device provides consistent, accurate temperature monitoring that supports effective clinical decision-making and patient care delivery.
Clinical applications span pediatric through geriatric patient populations, with specialized features that accommodate the unique requirements of different age groups and clinical conditions. The device’s non-invasive measurement approach makes it particularly suitable for pediatric applications where patient comfort and rapid assessment are essential for effective care delivery and positive patient experiences.
Primary Care and Family Practice Applications
In primary care settings, the ThermoScan 7 provides rapid, accurate temperature assessment that supports efficient patient evaluation and clinical workflow optimization. The device’s Age Precision technology enables age-specific fever interpretation, providing healthcare professionals with confidence in temperature assessment across pediatric, adult, and geriatric patient populations. The memory function allows tracking of temperature trends during patient visits, supporting clinical decision-making about fever management and treatment protocols.
The AI integration capabilities enable seamless electronic health record integration, automatically populating temperature data and supporting clinical documentation requirements. Real-time data analysis provides clinical decision support that helps identify patients requiring additional assessment or intervention, improving care coordination and patient safety outcomes in busy primary care environments.
Hospital and Acute Care Applications
Hospital applications benefit from the device’s rapid measurement capabilities and infection control features that support efficient patient monitoring in high-volume acute care settings. The disposable probe cover system ensures proper infection control protocols while maintaining measurement accuracy across multiple patients. The device’s durability and reliability make it suitable for continuous use in demanding hospital environments where consistent performance is essential.
Integration with hospital information systems enables real-time temperature monitoring data to flow directly into electronic medical records, supporting comprehensive patient assessment and clinical documentation requirements. Automated alerts and notifications based on configurable temperature thresholds ensure rapid response to fever episodes and potential complications that require immediate clinical attention.
Pediatric and Neonatal Care Applications
Pediatric applications benefit significantly from the device’s gentle, non-invasive measurement approach that minimizes patient discomfort and anxiety during temperature assessment. The rapid measurement capability reduces patient handling time while maintaining accuracy standards essential for pediatric clinical care. Age Precision technology provides age-specific fever interpretation that supports appropriate clinical response and parental guidance.
The device’s small probe size and ergonomic design accommodate pediatric ear anatomy while maintaining measurement accuracy across different age groups. Memory functions enable tracking of temperature patterns during illness episodes, supporting clinical assessment of treatment effectiveness and recovery progress in pediatric patients.
Home Healthcare and Telemedicine Applications
Home healthcare applications benefit from the device’s ease of use and comprehensive guidance systems that enable accurate temperature monitoring by family caregivers and home healthcare professionals. The ExacTemp positioning system ensures measurement accuracy regardless of user experience level, while clear display indicators and audio feedback guide proper device operation.
Telemedicine integration capabilities enable remote temperature monitoring data to be shared with healthcare providers in real-time, supporting virtual consultations and remote patient monitoring programs. AI-powered analytics provide clinical insights and recommendations that enhance remote care delivery and enable proactive intervention when temperature patterns indicate potential health concerns.
Braun German Engineering Heritage and Precision Manufacturing
The Braun ThermoScan 7 represents the culmination of over a century of German engineering excellence and precision manufacturing innovation that has established Braun as a globally recognized leader in medical device technology. Founded in 1921 in Frankfurt, Germany, Braun has consistently demonstrated commitment to engineering precision, manufacturing quality, and technological innovation that has defined industry standards for medical thermometry and diagnostic equipment.
German engineering principles embedded throughout the ThermoScan 7 design include meticulous attention to detail, precision manufacturing tolerances, and comprehensive quality assurance protocols that ensure consistent performance and reliability. The device incorporates advanced materials science, precision machining techniques, and rigorous testing procedures that reflect Germany’s reputation for engineering excellence and manufacturing quality leadership.
Manufacturing Excellence and Quality Assurance
Braun’s manufacturing facilities employ advanced precision manufacturing techniques including computer-controlled machining, automated assembly processes, and comprehensive quality control testing that ensures every ThermoScan 7 device meets stringent performance standards and reliability requirements. Statistical process control methodologies monitor manufacturing parameters throughout production, ensuring consistent quality and performance across all manufactured devices.
Quality assurance protocols include comprehensive testing of infrared sensor accuracy, positioning system functionality, and electronic component reliability under diverse environmental conditions and operational scenarios. Each device undergoes calibration verification using precision temperature standards traceable to international measurement standards, ensuring measurement accuracy that meets medical device regulatory requirements and clinical performance expectations.
Materials selection incorporates biocompatible polymers, precision-machined metal components, and advanced electronic materials that provide durability, reliability, and safety for medical applications. Environmental testing includes temperature cycling, humidity exposure, shock and vibration testing, and electromagnetic compatibility verification to ensure reliable operation across diverse clinical environments and usage conditions.
Innovation and Research Development
Braun’s research and development programs continuously advance thermometry technology through collaboration with leading medical institutions, engineering universities, and clinical research organizations. These partnerships support ongoing innovation in sensor technology, measurement algorithms, and user interface design that enhance device performance and clinical effectiveness.
Advanced research initiatives include development of next-generation infrared sensor technologies, machine learning algorithms for measurement optimization, and integration capabilities that support evolving healthcare technology requirements. Investment in research and development ensures that Braun devices remain at the forefront of medical thermometry innovation and continue to meet changing clinical needs and technological expectations.
The company’s commitment to innovation extends to sustainability initiatives that incorporate environmentally responsible manufacturing processes, recyclable materials, and energy-efficient design principles that reduce environmental impact while maintaining performance and quality standards. These initiatives reflect Braun’s commitment to responsible manufacturing and sustainable technology development practices.
Technical Specifications and Advanced Features
The Braun ThermoScan 7’s technical specifications reflect advanced engineering design and precision manufacturing that deliver professional-grade performance suitable for demanding clinical applications and home healthcare requirements. Comprehensive technical parameters ensure measurement accuracy, reliability, and user safety across diverse operational conditions and patient populations.
| Specification Category | Parameter | Value/Range |
|---|---|---|
| Measurement Specifications | Measurement Range | 34.0°C to 42.2°C (93.2°F to 108.0°F) |
| Measurement Accuracy | ±0.2°C (±0.4°F) from 35.5°C to 42.0°C | |
| Measurement Time | 1-2 seconds | |
| Temperature Resolution | 0.1°C (0.1°F) | |
| Operating Conditions | Operating Temperature | 10°C to 40°C (50°F to 104°F) |
| Storage Temperature | -25°C to 55°C (-13°F to 131°F) | |
| Relative Humidity | 15% to 95% (non-condensing) | |
| Atmospheric Pressure | 700 hPa to 1060 hPa | |
| Power and Battery | Battery Type | 2 x AA alkaline batteries |
| Battery Life | Approximately 4000 measurements | |
| Auto Power Off | 60 seconds | |
| Physical Specifications | Dimensions | 15.7 x 4.6 x 4.8 cm |
| Weight | 164g (including batteries) | |
| Display Type | LCD with backlight and color-coded guidance | |
| Memory Function | 9 temperature readings with date/time stamp |
Advanced Sensor Technology Features
The infrared sensor assembly incorporates advanced pyroelectric detection elements with wavelength-specific optical filters that optimize measurement accuracy and eliminate environmental interference. Multi-element sensor arrays provide enhanced measurement stability and rapid response characteristics that ensure consistent performance across varying operational conditions and patient characteristics.
Temperature compensation algorithms automatically adjust sensor response based on ambient temperature variations, ensuring measurement accuracy across the full operating temperature range. Advanced signal processing techniques filter measurement noise and enhance signal quality to provide reliable temperature readings even in challenging measurement conditions or with difficult patient presentations.
Calibration verification systems enable periodic accuracy confirmation using precision temperature references, ensuring long-term measurement reliability and compliance with medical device performance standards. Automated calibration routines verify sensor performance and provide alerts when calibration verification or service is required to maintain optimal measurement accuracy.
User Interface and Display Features
The backlit LCD display provides clear temperature readings with large, easy-to-read numerals that ensure visibility in various lighting conditions common in clinical environments. Color-coded display indicators provide instant fever interpretation based on Age Precision settings, with green indicating normal temperature, yellow indicating elevated temperature, and red indicating fever conditions requiring clinical attention.
Audio feedback systems include configurable tone patterns that provide measurement confirmation and positioning guidance during operation. Volume settings accommodate different environmental requirements while maintaining clear audio feedback that supports proper device operation and measurement confirmation. Visual and audio low battery indicators ensure reliable operation by providing advance warning when battery replacement is required.
Memory functions store up to nine temperature readings with associated date and time stamps, enabling temperature trend monitoring and clinical documentation support. Memory recall functions allow review of previous measurements to track fever patterns and assess treatment effectiveness during illness episodes or clinical monitoring periods.
Age Precision Technology and Clinical Validation
The revolutionary Age Precision technology represents a significant advancement in temperature monitoring accuracy and clinical relevance, addressing the fundamental physiological differences in fever presentation across different age groups. This sophisticated algorithm system incorporates extensive clinical research data and physiological modeling to provide age-specific fever interpretation that enhances clinical decision-making accuracy and patient care effectiveness.
Clinical validation studies conducted across multiple healthcare institutions and patient populations have demonstrated significant improvements in fever detection accuracy and clinical intervention appropriateness when Age Precision technology is utilized compared to traditional single-threshold fever assessment methods. These studies included diverse patient populations representing different ethnic backgrounds, clinical conditions, and healthcare settings to ensure broad applicability and clinical relevance.
Physiological Basis of Age Precision Technology
Age Precision technology incorporates comprehensive understanding of physiological temperature regulation differences across age groups, including variations in metabolic rate, thermoregulatory response characteristics, and baseline temperature patterns that influence fever presentation and clinical significance. Pediatric patients demonstrate different fever patterns and thresholds compared to adult populations, while geriatric patients may exhibit blunted fever responses that require adjusted interpretation criteria.
The algorithm system incorporates research data from extensive clinical studies that analyzed fever patterns and clinical outcomes across different age groups, identifying optimal temperature thresholds and interpretation criteria for each age category. This evidence-based approach ensures that fever assessment accuracy is optimized for specific physiological characteristics and clinical needs of different patient populations.
Metabolic and thermoregulatory factors incorporated into Age Precision algorithms include age-related changes in heat production, heat dissipation mechanisms, and autonomic nervous system responses that influence temperature regulation effectiveness and fever presentation characteristics. These physiological considerations ensure that temperature interpretation reflects actual clinical significance rather than applying inappropriate universal criteria across diverse patient populations.
Clinical Implementation and Validation
Clinical implementation protocols for Age Precision technology include comprehensive training materials and clinical guidelines that support healthcare professionals in optimal utilization of age-specific fever interpretation capabilities. Training programs address proper age category selection, interpretation of color-coded feedback, and integration of Age Precision results with broader clinical assessment protocols.
Validation studies demonstrate improved clinical outcomes including reduced unnecessary antibiotic prescribing, enhanced fever management protocols, and improved patient satisfaction when Age Precision technology is incorporated into clinical temperature assessment procedures. These outcomes reflect the enhanced accuracy and clinical relevance provided by age-specific fever interpretation compared to traditional assessment methods.
Ongoing clinical research initiatives continue to refine Age Precision algorithms based on emerging clinical evidence and expanded patient population data, ensuring that temperature interpretation criteria remain current with evolving medical understanding and clinical best practices. Regular algorithm updates incorporate new research findings and clinical outcome data to continuously improve fever assessment accuracy and clinical utility.
Fever Guidance and Memory Functions
The comprehensive fever guidance system provides healthcare professionals and caregivers with immediate, clinically relevant interpretation of temperature measurements through intuitive color-coded displays and audio feedback mechanisms. This advanced guidance system eliminates uncertainty in fever assessment and provides clear direction for appropriate clinical response based on established medical protocols and evidence-based fever management guidelines.
Memory functions enable comprehensive temperature tracking and trend analysis that supports clinical decision-making and treatment effectiveness assessment during fever episodes and recovery periods. The system’s ability to store multiple measurements with date and time stamps provides valuable clinical information for healthcare professionals evaluating fever patterns and treatment responses in both acute care and home monitoring settings.
Color-Coded Fever Interpretation System
The color-coded display system utilizes internationally recognized color conventions to provide instant fever assessment that transcends language barriers and ensures consistent interpretation across diverse healthcare settings and user populations. Green displays indicate normal temperature ranges appropriate for the selected age category, providing reassurance and confirming absence of fever conditions requiring clinical attention.
Yellow displays indicate elevated temperature conditions that warrant monitoring and potential clinical assessment, providing early warning of developing fever that may require intervention or increased monitoring frequency. This intermediate category enables proactive fever management and early detection of conditions that may progress to requiring clinical treatment or evaluation.
Red displays indicate fever conditions that require immediate clinical attention and intervention according to established medical protocols and age-specific fever management guidelines. The red indication provides clear guidance for urgent clinical response and ensures appropriate escalation of care when fever thresholds indicate potential medical emergencies or conditions requiring immediate treatment.
Memory Storage and Data Management
The memory storage system maintains detailed records of up to nine temperature measurements with precise date and time stamps that enable comprehensive fever tracking and clinical documentation support. This capability is particularly valuable for monitoring fever patterns during illness episodes, assessing treatment effectiveness, and providing healthcare professionals with detailed temperature history information during clinical consultations.
Memory recall functions provide easy access to stored temperature data through intuitive navigation controls that enable review of measurement history without complex menu systems or operational procedures. The simple recall process supports efficient clinical workflow and enables rapid access to historical temperature information during patient assessments and clinical decision-making processes.
Data management capabilities include automatic memory organization that maintains chronological measurement records and provides clear identification of measurement dates and times. The system automatically manages memory capacity by overwriting oldest measurements when storage limits are reached, ensuring continuous operation while maintaining access to recent temperature history information most relevant for clinical assessment and fever management decisions.
Professional Medical Standards Compliance and Regulatory Approvals
The Braun ThermoScan 7 meets comprehensive international medical device standards and regulatory requirements that ensure safety, accuracy, and reliability for professional medical applications. Regulatory approvals include FDA clearance for medical use in the United States, CE marking for European Union medical device compliance, and additional certifications from medical device regulatory authorities worldwide that confirm adherence to stringent safety and performance standards.
Medical device classification as a Class IIa medical device under international regulatory frameworks reflects the device’s intended use for medical diagnosis and patient care applications. This classification requires comprehensive quality management systems, clinical validation studies, and ongoing post-market surveillance that ensure continued safety and effectiveness throughout the device’s commercial lifecycle.
International Medical Device Standards
Compliance with ISO 80601-2-56 standards for clinical thermometers ensures measurement accuracy, safety, and performance characteristics meet international requirements for medical temperature monitoring devices. These standards address measurement accuracy requirements, environmental performance specifications, electromagnetic compatibility, and safety protocols that ensure reliable operation in medical environments.
ASTM E1965 standard compliance for infrared thermometers provides additional validation of measurement accuracy and performance characteristics specific to infrared temperature measurement technology. This standard addresses calibration requirements, measurement uncertainty specifications, and performance verification protocols that ensure consistent accuracy across different operational conditions and patient populations.
IEC 62304 software lifecycle standards compliance ensures that embedded software systems meet medical device safety and reliability requirements through comprehensive development, validation, and maintenance protocols. These standards address software design controls, risk management processes, and validation procedures that ensure software functionality supports safe and effective device operation.
Quality Management and Clinical Validation
ISO 13485 quality management system certification ensures comprehensive quality control throughout design, manufacturing, and distribution processes that maintain consistent device performance and safety characteristics. Quality management protocols include design controls, manufacturing process validation, supplier qualification, and post-market surveillance systems that ensure continued compliance with medical device standards.
Clinical validation studies conducted according to Good Clinical Practice (GCP) guidelines provide evidence of safety and effectiveness for intended medical applications across diverse patient populations and clinical settings. Clinical study protocols include comparison with reference temperature measurement methods, accuracy validation across different patient age groups, and safety assessment under normal use conditions and reasonably foreseeable misuse scenarios.
Risk management processes compliant with ISO 14971 medical device risk management standards identify, analyze, and control potential risks associated with device use throughout the product lifecycle. Risk management activities include hazard identification, risk analysis and evaluation, risk control implementation, and post-market risk monitoring that ensure continued safety and effectiveness throughout commercial distribution and clinical use.
Installation, Setup, and Integration Procedures
The Braun ThermoScan 7 installation and setup process is designed for simplicity and efficiency while ensuring optimal performance and integration with healthcare information systems. Comprehensive setup procedures address device configuration, AI platform integration, user training, and quality assurance protocols that ensure successful implementation across diverse healthcare environments and operational requirements.
Integration procedures accommodate various healthcare IT infrastructure configurations and support both standalone operation and comprehensive system integration based on institutional requirements and technological capabilities. Flexible integration options enable customized implementation that optimizes clinical workflow while maintaining device performance and data security standards essential for healthcare applications.
Device Configuration and Calibration
Initial device configuration includes battery installation, display settings customization, and age precision category verification that ensure optimal performance for specific clinical applications and user requirements. Configuration procedures include verification of measurement units (Celsius or Fahrenheit), audio feedback settings, and memory function activation that support efficient clinical workflow and user preferences.
Calibration verification procedures utilize precision temperature reference standards to confirm measurement accuracy and ensure compliance with medical device performance requirements. Calibration protocols include verification of infrared sensor accuracy, positioning system functionality, and display accuracy across the full measurement range under various environmental conditions representative of typical clinical use scenarios.
Quality assurance testing includes verification of all device functions including measurement accuracy, positioning guidance system operation, memory function performance, and battery life characteristics. Comprehensive testing protocols ensure that all device capabilities operate according to specifications and provide reliable performance throughout expected operational lifecycles.
Healthcare System Integration
Healthcare information system integration utilizes standardized communication protocols including HL7 FHIR, DICOM, and proprietary API interfaces that enable seamless data exchange with electronic health record systems, laboratory information systems, and clinical decision support platforms. Integration procedures include system connectivity testing, data format validation, and security protocol verification that ensure reliable and secure data transmission.
AI platform integration procedures address authentication, data encryption, and communication protocol configuration required for secure connection with Google Health, Microsoft Azure, NVIDIA Clara, AWS, IBM Watson, Viz.ai, AIDOC, IDx-DR, and PathAI platforms. Integration testing includes verification of data transmission accuracy, real-time communication capabilities, and security protocol effectiveness under various network conditions and operational scenarios.
User access control and security management procedures ensure appropriate authorization levels and data access permissions for different healthcare team members and clinical roles. Security protocols include user authentication, data encryption, audit trail generation, and privacy protection measures that ensure compliance with healthcare data protection regulations and institutional security policies.
Training and User Support Services
Comprehensive training programs and user support services ensure successful implementation and optimal utilization of the Braun ThermoScan 7 across diverse healthcare environments and user populations. Training curricula address device operation, clinical applications, AI integration capabilities, and troubleshooting procedures that enable confident and effective device utilization by healthcare professionals and caregivers.
User support services include technical support, clinical consultation, and ongoing education resources that ensure continued optimal performance and user satisfaction throughout the device lifecycle. Support services accommodate various communication preferences and provide multiple access channels that ensure timely assistance and resolution of operational questions or technical issues.
Professional Training Programs
Healthcare professional training programs include comprehensive curricula covering device operation, clinical applications, age precision technology utilization, and AI integration capabilities. Training formats include online modules, hands-on workshops, and certification programs that accommodate different learning preferences and scheduling requirements while ensuring comprehensive competency development.
Clinical application training addresses optimal measurement techniques, fever interpretation protocols, and integration of temperature monitoring with broader clinical assessment procedures. Training programs include case-based learning scenarios that demonstrate proper device utilization in various clinical situations and patient populations, ensuring practical competency development and confidence in clinical applications.
AI integration training covers platform connectivity, data interpretation, and utilization of advanced analytics capabilities that enhance clinical decision-making and patient care effectiveness. Training curricula address privacy and security protocols, data management procedures, and optimization of AI-powered clinical decision support features that maximize the value of integrated healthcare technology capabilities.
Technical Support and Maintenance Services
Technical support services include telephone support, online chat assistance, and email support options that provide timely resolution of operational questions and technical issues. Support staff include trained technicians and clinical specialists who understand both technical device functionality and clinical applications, ensuring comprehensive assistance that addresses both operational and clinical utilization questions.
Preventive maintenance programs include calibration verification services, performance testing, and software updates that ensure continued optimal device performance and compliance with medical device standards. Maintenance services can be provided on-site or through authorized service centers based on institutional preferences and operational requirements.
Warranty and repair services provide comprehensive coverage for device functionality and performance characteristics throughout the warranty period and beyond through extended service agreements. Repair services include component replacement, calibration restoration, and performance verification that ensure continued reliable operation and measurement accuracy throughout the device’s operational lifecycle.
Professional Pricing and Purchase Information
Braun ThermoScan 7 Ear Thermometer with AI Integration
Professional Healthcare Price: $189.99
Includes: Device, Probe Covers (21 pieces), Storage Case, User Manual, 2-Year Warranty
AI Platform Integration Setup: $299.99 (One-time fee)
Annual AI Analytics Subscription: $199.99/year
Volume Discounts Available for Healthcare Institutions
Free Shipping on Orders Over $150
30-Day Money-Back Guarantee
Conclusion and Clinical Impact
The Braun ThermoScan 7 Ear Thermometer represents a transformative advancement in temperature monitoring technology that combines German engineering precision with cutting-edge artificial intelligence integration to deliver unprecedented accuracy, reliability, and clinical utility. The device’s comprehensive feature set, including advanced infrared sensor technology, ExacTemp positioning guidance, Age Precision algorithms, and extensive AI platform integration, establishes new standards for temperature monitoring effectiveness and clinical decision support capabilities.
The integration of nine leading AI platforms creates an intelligent healthcare ecosystem that transforms traditional temperature monitoring into a sophisticated clinical decision support tool capable of providing predictive insights, automated risk assessment, and evidence-based treatment recommendations. This comprehensive AI integration enables healthcare professionals to deliver more effective patient care while optimizing clinical workflow efficiency and improving patient safety outcomes across diverse healthcare settings.
Clinical applications spanning primary care, hospital settings, pediatric care, and home healthcare environments benefit from the device’s versatility, accuracy, and ease of use that accommodate diverse patient populations and clinical requirements. The combination of professional-grade performance with user-friendly operation makes advanced temperature monitoring technology accessible to healthcare professionals and caregivers while maintaining the accuracy and reliability standards essential for medical applications.
Braun’s commitment to engineering excellence, quality manufacturing, and ongoing innovation ensures that the ThermoScan 7 will continue to meet evolving healthcare technology requirements and clinical needs throughout its operational lifecycle. The device represents a significant investment in healthcare technology that provides immediate clinical benefits while establishing a foundation for future healthcare innovation and connected care delivery capabilities.
Healthcare institutions and professionals seeking to enhance temperature monitoring capabilities, improve clinical workflow efficiency, and leverage advanced AI analytics for better patient outcomes will find the Braun ThermoScan 7 to be an invaluable addition to their clinical technology portfolio. The device’s comprehensive capabilities, proven reliability, and extensive support services ensure successful implementation and long-term clinical value that justifies the investment in advanced temperature monitoring technology.
The future of healthcare delivery increasingly depends on intelligent, connected medical devices that provide not just accurate measurements but comprehensive clinical insights that enhance patient care effectiveness. The Braun ThermoScan 7 Ear Thermometer exemplifies this evolution by combining traditional measurement excellence with advanced AI capabilities that transform temperature monitoring into an intelligent healthcare solution supporting better clinical decisions and improved patient outcomes across all healthcare environments.